In the last few articles in this series, we have discussed a lot about diet quantity, and I want this article to serve as a kind of overview of diet quantity. This will allow you to quickly reference back to the most important information, without having to go through the more in-depth articles every time.
I have also included a few further thoughts on diet quantity, that don’t neatly fit into the rest of the articles, but will help you to better understand diet quantity and how to actually set up and follow a healthy diet.
Before we get stuck in, I would just like to remind you that we offer comprehensive online coaching. So if you need help with your own exercise program or nutrition, don’t hesitate to reach out. If you are a coach (or aspiring coach) and want to learn how to coach nutrition, then consider signing up to our Nutrition Coach Certification course. We do also have an exercise program design course in the works, if you are a coach who wants to learn more about effective program design and how to coach it. We do have other courses available too.
Table of Contents
Diet Quantity: Calories and Macronutrients Summary
We have covered a lot in this article series on setting up the diet. So far in the article series, we have discussed setting up the calories for the diet, how much protein should you eat, how much fat should you eat, how much carbohydrate you should eat, how much fibre you should eat, how much water you should drink and dealing with alcohol in the diet.
Theres a lot in there, and I am confident that if you read through those article, you will have a fairly comprehensive understanding of nutrition. However, I also know that you want somewhere to quickly reference back to just the most important points.
So let’s just quickly recap things. When we are discussing diet quantity, the targets are as follows:
- Calories: Set appropriately based on your goals (deficit, surplus, maintenance).
- Protein: Aim for 1.8-2.2g per kg of protein per day, spread out across the day relatively evenly.
- Fat: Aim for 0.8-1g per kg of fats per day, with 1-5g of EPA/DHA per day, and less than 10% of total calories as saturated fat.
- Carbs: The rest of your available calories after you have set your protein and fat targets should be allotted to carbohydrates. Carbohydrate intake should preferentially be obtained from complex carbohydrates rather than simple carbohydrates.
- Fibre: Set your fibre target somewhere in the range of 10-15g per 1000 calories.
- Water: Consume somewhere around 40mL of water per kg or 1.5mL of water per calorie, and refine this intake and distribution so that you are urinating relatively clear ~5+ times per day.
- Alcohol: Ideally, consume zero alcohol, but if you must consume it, aim to consume less than 14 units of alcohol per week, spread out over at least 3 non-consecutive days. Account for the calories of the drinks you consume, substituting out fats and/or carbs to allow for the alcohol intake.
There are obviously caveats and further refinements, but that is what the in-depth articles are for. This is just the key information, refined down as simple as I can make it while still allowing you to actually design an effective diet.
You can use our diet set up calculator or our calorie and macronutrient calculator to help you to calculate your targets for you.
Now, we didn’t discuss this too much (although we touched on it while discussing metabolism), but it also makes sense to try and somewhat standardise your non-exercise activity thermogenesis (NEAT). To do this, we generally just set a daily step count target.
While the figure of 10,000 steps per day gets thrown around a lot, it doesn’t actually need to be this number, and we really just want to try and standardise things to some extent. So a daily step count somewhere in the range of 5,000 to 15,000 steps per day is a good idea.
Pick a number that makes sense to you and your life situation, and try to stay relatively consistent with it.
You can set a weekly target, however, very often people will then remain very inactive Monday to Friday and they try to get 50,000 steps (or whatever ridiculously high number) over the course of the weekend. This is usually very hard to do, and it likely will leave you very fatigued. So while you can set a weekly target, we do still want to relatively evenly spread our step count out across the week.

Diet Priorities
That’s how you set up a diet, from a diet quantity perspective at least. There is more to the diet than purely diet quantity, but we will cover that in further articles.
The beauty of these diet quantity targets is in their simplicity, the hard work is in the actual implementation.
Be under no delusion, dietary adherence can be very hard for some people and there may be numerous barriers in the way of you even being able to eat in alignment with these targets.
Ensuring adherence and actual calorie tracking methods are as accurate as possible is tough, and you should expect that there are going to be many ups and downs along the way. Just because you know what your targets should be, doesn’t mean you will actually be able to consistently achieve them.
One way we have found to really help with this is knowing what your priorities are with the diet itself. If you know what is more important, you can at least focus on the more important things, even if you can’t get everything “perfect”.
In terms of the diet itself, the order of priorities are:
- Calories
- Protein
- Energy substrates (carbs and fats)
- Other recommendations
So the most important thing is to consume a calorie-appropriate diet. Even if the rest of the diet is a bit all over the place, if you consume the right amount of calories, you will usually be able to still move towards your health, body composition and performance goals.
After that, protein intake is the next most important thing. If you can get your protein intake where it needs to be, then this usually has a very positive impact on your diet overall. It also usually allows you to really progress nicely towards your health, body composition and performance goals. If all you did was set a calorie target and a protein target, you would actually get incredibly far with your diet.
After that, carbs and fats are pretty equal. While we have given specific targets for both, you can actually get away with quite a range with intake. Higher and lower fat/carb intakes can work.
While we think the targets we have outlined make the most sense for the vast majority of people, as long as you stay within your calories and hit your protein target, you do actually have a good bit of leeway with carbs/fats.
If you are trying to optimise things, then obviously you are going to have to be closer to optimal intakes, however, if you just want to eat a “good enough” diet, then you do have quite a bit of wiggle room.
After that, the other various recommendations are the priority. Saturated fat intake is one that I would personally prioritise, as heart disease is one of the biggest threats to your life, but if you follow good food selection practices (outlined in further articles in this series), this does actually look after itself quite easily. Omega-3 intake is also relatively easily looked after with good food practices, as is fibre intake, so you can prioritise them less.
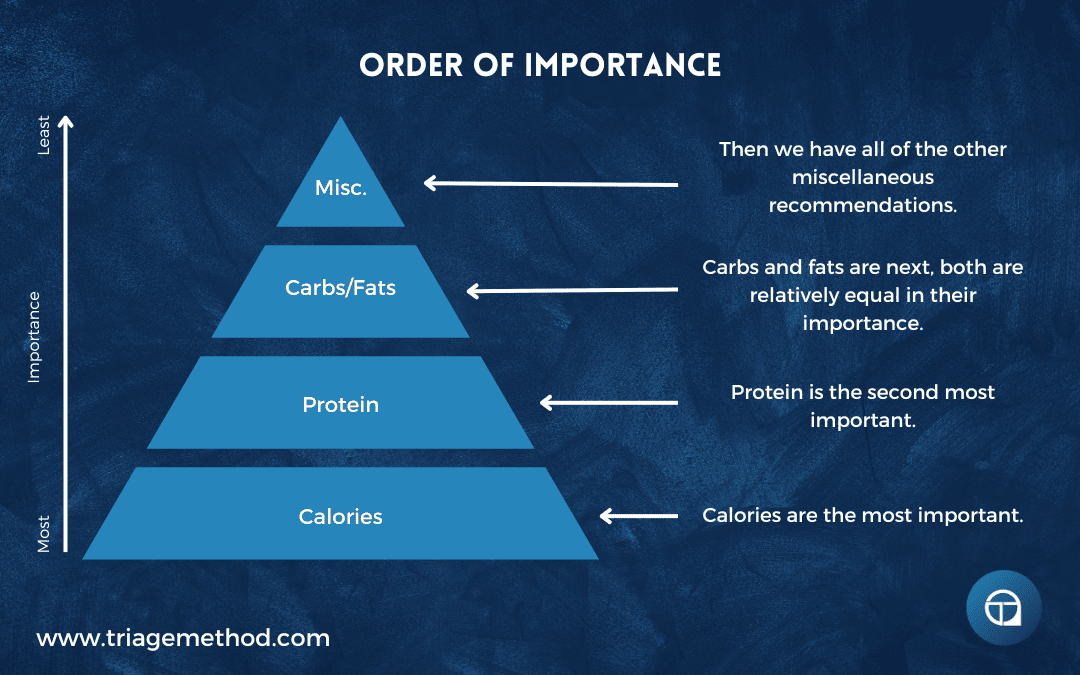
Of course, the ideal would be to prioritise everything, but this can be overwhelming at first, and in reality, nobody ever gets the diet perfect anyway. But if you can get your calories dialled in, and then eat enough protein, you will usually have put yourself in a really good position with the diet.
For certain populations or goals, this order of importance may be changed (for example, someone at a high risk of heart disease may need to put a bigger priority on lowering saturated fat and salt intake than someone looking to lose a few kilos), but this generally holds true as a baseline order of importance.
Ranges vs. Set Targets
Another thing that can make the diet much easier is to realise that there is actually quite a lot of leeway with the numbers. As you can see, with all our recommendations, there is actually a broad range of what you could actually set your macronutrient targets as.
There are minimum numbers, more optimal numbers and then higher numbers. As long as your minimum targets are met you will have a better diet setup than the vast majority of people.
Of course, you may want to really try to nail optimal, and you can aim for those numbers then.
Perhaps you just really like a certain macronutrient, and as a result, you may want to consume a bit more of that macronutrient, even if it means you get to eat less of another one as a result.
So what we very often suggest is having a rough range for your macronutrient intakes.
Know what the bare minimum is, and know where the more optimal number is. Some days you will be more dialled in, and other days will be more of a struggle. But in general, unless you are trying to get super lean under a certain time constraint (like a bodybuilding competition or photoshoot) or you are an athlete trying to milk every last improvement you can possibly get from the diet, shooting for macronutrient ranges is actually much better than aiming for an absolute fixed macronutrient target.
So for example using that theoretical 70kg person with a maintenance of 2000 calories, protein would be set at a range of 126-154 per day and then we can play around a little bit more with the fat and carbohydrate ranges. We could easily set the range of fats to 56-70g per day and then we would just eat the rest as carbohydrates.
Alternatively, you could set a carb minimum and then eat fats and carbs to fulfil whatever calories remain. If you eat more carbohydrates than your macronutrient targets dictate, you can simply lower your fat intake to compensate, and vice versa.
There is a lot of flexibility in this. However, what can very often happen is that when people use ranges, they find themselves under-consuming protein or not staying within their calorie target.
So if we are going to use ranges, we have to still keep the priorities of the diet in mind and we still need to have some structure to the diet.
If you are constantly missing your targets because you are using ranges, you need to rethink whether your targets are manageable. You also need to assess whether you are actually planning your diet out accordingly and not just “winging it” and hoping you hit your targets by the end of the day.
This very often happens, and you will see people try to consume their entire protein target at the end of the day, as they have simply not prioritised it during the day. This rarely works.
How Dialled In With The Targets Do You Have To Be?
I should also mention that you don’t always need to be perfect with your targets. There is some degree of wiggle room built into the system, and humans are survival machines.
So don’t get too caught up in trying to be perfect with the numbers, even if you are someone trying to be more optimised with your diet.
“In and around” is generally good enough, once you stay roughly within your calorie and protein targets.
Keep the order of priorities in mind, and try to come close to your targets, but don’t sweat it if you are slightly out.
Now, if you are consistently not hitting your targets, especially if it is always below or above, then you may need to reassess either the targets or the way you are trying to actually implement the diet.
But in most cases, once you follow generally sound diet practices, being in and around the targets should be good enough.
Individual Differences
It is also important to note that there is still a lot of individual difference that needs to be taken into account with the diet.
Some people feel fuller for longer with a higher fat intake, while others feel fuller with a higher carbohydrate intake. Protein is generally the most satiating of all the macronutrients. However, you have to take into account the subtle nuances of how your diet affects you as an individual.
So after setting initial numbers, you must constantly assess how eating meals to achieve these numbers actually affects you.
Perhaps you notice that eating carbohydrates for breakfast leaves you feeling tired and groggy for the rest of the day, or you experience that post-lunch slump when you eat a certain meal composition. Well, we need to reevaluate that meal.
The numbers may fit your overall day, but this must take into account how that meal breakdown affects your performance. So if you notice eating carbohydrates earlier in the day leaves you feeling lower in energy, then you can either adjust your intake to a higher fat intake, or you can move more of your carbs to later in the day.
Those of you with higher activity levels (you have a more physical job, you walk a lot, etc.) will likely find yourself performing better with a higher carbohydrate intake, while those who are more sedentary may find that a higher fat intake suits you better.
However, probably the biggest factor that affects the individuality of your diet, is preference. Adherence is the most important part of actually achieving the results you desire, so if you can’t sustain the numbers that you have chosen because it stops you from eating the foods you prefer, then you won’t be able to adhere long term.
If you prefer eating foods that are higher in fat, set your fat intake higher and set carbohydrates lower, and vice versa.
It doesn’t have to be an all-the-time thing either. If you notice that you do actually perform better with a higher carbohydrate, lower fat diet, but you have a hot date and they want to go get a meal that is going to be higher in fat and lower in carbohydrates, adjust your number for that day. Long-term adherence is more important than transient dietary “perfection”.
Making Adjustments
There will come a time, even if you set the diet up “perfectly” to start with, when you will need to adjust the diet. This could be because results are as predicted, or have slowed, your circumstances or goals have changed or a variety of other reasons. So how do we adjust the diet?
The main variable to adjust, for most goals, is calories. When we make calorie changes, we generally want to make small changes, rather than large changes. Unless you have drastically changed your activity habits, and now need a lot more food to even maintain your body weight, more often than not, we are only going to make calorie changes in the order of 200-500 calories.
If weight loss/gain has stalled, for example, we might adjust calories by ~300 calories and then wait and see how the body responds.
Generally, we will also wait at least 2-3 weeks to allow the dietary changes to actually manifest in bodily changes. It is very rare that you would need to make drastic calorie changes, and it would be even rarer that you would need to make drastic calorie changes on a very frequent basis.
If things are moving in the right direction at an acceptable rate, you don’t need to make changes.
Now, what macronutrients would you adjust to make these calorie changes? We will generally keep protein intake fairly stable, and any changes will generally come from fat or carbs.
If fat loss is the goal, for most people, it is easiest to change fat intake first and foremost. This way you keep carbs higher, and thus reduce muscle loss and keep performance high.
When trying to lose fat, we will generally reduce fat intake, as dictated by the calorie reductions. So if you were dropping 200 calories, we would reduce fat intake by ~22g. However, we generally would move to carbs for any calorie reductions once fat intake reached the bottom end of the threshold (<0.6g of fat per kg).
Conversely, if you were trying to gain weight, we would prioritise calorie increases from fat until you were at least consuming ~0.8g per kg. Once you are consuming that much, we would generally prioritise any additional calories to be increased via carb intake.
In practice, it is rare that you would just change only one macronutrient, and it will generally be a mix of carbs and fats. This is in accordance with the priorities of the diet, and once you are at least consuming the minimum intake recommendations for fat, you do have quite a lot of wiggle room.
However, it should be noted that for most athletic, performance and muscle gain goals, carbs are likely going to be the biggest bang for your buck macronutrient (assuming protein requirements are met).
Closing Thoughts on Diet Quantity

So there you have it, a nice and neat outline of how the diet quantity aspect of nutrition. It is straightforward enough, at least in theory. In practice, it can be quite difficult to implement.
If you need help with this, you can always reach out to us and get online coaching, or alternatively, you can interact with our free content.
If you want more free information on nutrition, you can follow us on Instagram, YouTube or listen to the podcast, where we discuss all the little intricacies of exercise and nutrition. You can always stay up to date with our latest content by subscribing to our newsletter.
Finally, if you want to learn how to coach nutrition, then consider our Nutrition Coach Certification course, and if you want to learn to get better at exercise program design, then consider our course on exercise program design. We do have other courses available too. If you don’t understand something, or you just need clarification, you can always reach out to us on Instagram or via email.
The previous article in this series is about Dealing With Alcohol In The Diet and the next article in this series is Understanding Diet Quality, if you are interested in continuing to learn about nutrition. You can also go to our nutrition hub to find more nutrition content.
References and Further Reading
Fell, D. A., & Thomas, S. (1995). Physiological control of metabolic flux: the requirement for multisite modulation. Biochemical Journal, 311(1), 35–39. http://doi.org/10.1042/bj3110035
Levine, J. A. (2002). Non-exercise activity thermogenesis (NEAT). Best Practice & Research Clinical Endocrinology & Metabolism, 16(4), 679–702. http://doi.org/10.1053/beem.2002.0227
Ballesteros, F. J., Martinez, V. J., Luque, B., Lacasa, L., Valor, E., & Moya, A. (2018). On the thermodynamic origin of metabolic scaling. Scientific Reports, 8(1). http://doi.org/10.1038/s41598-018-19853-6
Manini, T. M. (2010). Energy expenditure and ageing. Ageing Research Reviews, 9(1), 1–11. http://doi.org/10.1016/j.arr.2009.08.002
Mcmurray, R. G., Soares, J., Caspersen, C. J., & Mccurdy, T. (2014). Examining Variations of Resting Metabolic Rate of Adults. Medicine & Science in Sports & Exercise, 46(7), 1352–1358. http://doi.org/10.1249/mss.0000000000000232
Stiegler, P., & Cunliffe, A. (2006). The Role of Diet and Exercise for the Maintenance of Fat-Free Mass and Resting Metabolic Rate During Weight Loss. Sports Medicine, 36(3), 239–262. http://doi.org/10.2165/00007256-200636030-00005
Curtis, V., Henry, C. J. K., Birch, E., & Ghusain-Choueiri, A. (1996). Intraindividual variation in the basal metabolic rate of women: Effect of the menstrual cycle. American Journal of Human Biology, 8(5), 631–639. http://doi.org/10.1002/(sici)1520-6300(1996)8:5<631::aid-ajhb8>3.0.co;2-y
Harris, J. A., & Benedict, F. G. (1918). A Biometric Study of Human Basal Metabolism. Proceedings of the National Academy of Sciences, 4(12), 370–373. http://doi.org/10.1073/pnas.4.12.370
Roza, A. M., & Shizgal, H. M. (1984). The Harris-Benedict equation reevaluated: resting energy requirements and the body cell mass. The American Journal of Clinical Nutrition, 40(1), 168–182. http://doi.org/10.1093/ajcn/40.1.168
Mifflin, M. D., Jeor, S. T. S., Hill, L. A., Scott, B. J., Daugherty, S. A., & Koh, Y. O. (1990). A new predictive equation for resting energy expenditure in healthy individuals. The American Journal of Clinical Nutrition, 51(2), 241–247. http://doi.org/10.1093/ajcn/51.2.241
Frankenfield, D., Roth-Yousey, L., & Compher, C. (2005). Comparison of Predictive Equations for Resting Metabolic Rate in Healthy Nonobese and Obese Adults: A Systematic Review. Journal of the American Dietetic Association, 105(5), 775–789. http://doi.org/10.1016/j.jada.2005.02.005
Johnstone, A. M., Murison, S. D., Duncan, J. S., Rance, K. A., & Speakman, J. R. (2005). Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. The American Journal of Clinical Nutrition, 82(5), 941–948. http://doi.org/10.1093/ajcn/82.5.941
Speakman, J. R., Król, E., & Johnson, M. S. (2004). The Functional Significance of Individual Variation in Basal Metabolic Rate. Physiological and Biochemical Zoology, 77(6), 900–915. http://doi.org/10.1086/427059
Smith, D. A., Dollman, J., Withers, R. T., Brinkman, M., Keeves, J. P., & Clark, D. G. (1997). Relationship between maximum aerobic power and resting metabolic rate in young adult women. Journal of Applied Physiology, 82(1), 156–163. http://doi.org/10.1152/jappl.1997.82.1.156
Ravussin, E., Lillioja, S., Anderson, T. E., Christin, L., & Bogardus, C. (1986). Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation, 78(6), 1568–1578. http://doi.org/10.1172/jci112749
Wolfe, R. R. (2006). The underappreciated role of muscle in health and disease. The American Journal of Clinical Nutrition, 84(3), 475–482. http://doi.org/10.1093/ajcn/84.3.475
Wang, Z., Heshka, S., Zhang, K., Boozer, C. N., & Heymsfield, S. B. (2001). Resting Energy Expenditure: Systematic Organization and Critique of Prediction Methods*. Obesity, 9(5), 331–336. http://doi.org/10.1038/oby.2001.42
Mcpherron, A. C., Guo, T., Bond, N. D., & Gavrilova, O. (2013). Increasing muscle mass to improve metabolism. Adipocyte, 2(2), 92–98. http://doi.org/10.4161/adip.22500
Levine, J. A., Weg, M. W. V., Hill, J. O., & Klesges, R. C. (2006). Non-Exercise Activity Thermogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(4), 729–736. http://doi.org/10.1161/01.atv.0000205848.83210.73 http://www.fao.org/3/m2845e/m2845e00.htm
Martin, C. K., Heilbronn, L. K., Jonge, L. D., Delany, J. P., Volaufova, J., Anton, S. D., … Ravussin, E. (2007). Effect of Calorie Restriction on Resting Metabolic Rate and Spontaneous Physical Activity**. Obesity, 15(12), 2964–2973. http://doi.org/10.1038/oby.2007.354
Redman, L. M., Heilbronn, L. K., Martin, C. K., Jonge, L. D., Williamson, D. A., Delany, J. P., & Ravussin, E. (2009). Metabolic and Behavioral Compensations in Response to Caloric Restriction: Implications for the Maintenance of Weight Loss. PLoS ONE, 4(2). http://doi.org/10.1371/journal.pone.0004377
Martin, C. K., Das, S. K., Lindblad, L., Racette, S. B., Mccrory, M. A., Weiss, E. P., … Kraus, W. E. (2011). Effect of calorie restriction on the free-living physical activity levels of nonobese humans: results of three randomized trials. Journal of Applied Physiology, 110(4), 956–963. http://doi.org/10.1152/japplphysiol.00846.2009
Stiegler, P., & Cunliffe, A. (2006). The Role of Diet and Exercise for the Maintenance of Fat-Free Mass and Resting Metabolic Rate During Weight Loss. Sports Medicine, 36(3), 239–262. http://doi.org/10.2165/00007256-200636030-00005
Goran, M. I. (2005). Estimating energy requirements: regression based prediction equations or multiples of resting metabolic rate. Public Health Nutrition, 8(7a), 1184–1186. http://doi.org/10.1079/phn2005803
Johannsen, D. L., Knuth, N. D., Huizenga, R., Rood, J. C., Ravussin, E., & Hall, K. D. (2012). Metabolic Slowing with Massive Weight Loss despite Preservation of Fat-Free Mass. The Journal of Clinical Endocrinology & Metabolism, 97(7), 2489–2496. http://doi.org/10.1210/jc.2012-1444
Clamp, L., Hume, D., Lambert, E., & Kroff, J. (2018). Successful and unsuccessful weight-loss maintainers: Strategies to counteract metabolic compensation following weight loss. Journal of Nutritional Science, 7, E20. doi:10.1017/jns.2018.11 https://doi.org/10.1017/jns.2018.11
Hall, K. D. (2018). The complicated relation between resting energy expenditure and maintenance of lost weight. The American Journal of Clinical Nutrition, 108(4), 652–653. https://doi.org/10.1093/ajcn/nqy259
Ostendorf, D. M., Melanson, E. L., Caldwell, A. E., Creasy, S. A., Pan, Z., MacLean, P. S., Wyatt, H. R., Hill, J. O., & Catenacci, V. A. (2018). No consistent evidence of a disproportionately low resting energy expenditure in long-term successful weight-loss maintainers. The American Journal of Clinical Nutrition, 108(4), 658–666. https://doi.org/10.1093/ajcn/nqy179
Heilbronn, L. K., Jonge, L. D., Frisard, M. I., Delany, J. P., Larson-Meyer, D. E., Rood, J., … Team, F. T. P. C. (2006). Effect of 6-Month Calorie Restriction on Biomarkers of Longevity, Metabolic Adaptation, and Oxidative Stress in Overweight Individuals. Jama, 295(13), 1539. http://doi.org/10.1001/jama.295.13.1539
Zurlo, F., Trevisan, C., Vitturi, N., Ravussin, E., Salvò, C., Carraro, S., … Avogaro, A. (2019). One-year caloric restriction and 12-week exercise training intervention in obese adults with type 2 diabetes: emphasis on metabolic control and resting metabolic rate. Journal of Endocrinological Investigation, 42(12), 1497–1507. http://doi.org/10.1007/s40618-019-01090-x
Gilliat-Wimberly, M., Manore, M. M., Woolf, K., Swan, P. D., & Carroll, S. S. (2001). Effects of Habitual Physical Activity on the Resting Metabolic Rates and Body Compositions of Women Aged 35 to 50 Years. Journal of the American Dietetic Association, 101(10), 1181–1188. http://doi.org/10.1016/s0002-8223(01)00289-9
Pontzer H, Durazo-Arvizu R, Dugas LR, et al. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr Biol. 2016;26(3):410-417. doi:10.1016/j.cub.2015.12.046 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4803033/
Pontzer H. Constrained Total Energy Expenditure and the Evolutionary Biology of Energy Balance. Exerc Sport Sci Rev. 2015;43(3):110-116. doi:10.1249/JES.0000000000000048 https://pubmed.ncbi.nlm.nih.gov/25906426/
Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the Female Athlete Triad–Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(7):491-497. doi:10.1136/bjsports-2014-093502 https://pubmed.ncbi.nlm.nih.gov/24620037/
Mountjoy M, Sundgot-Borgen JK, Burke LM, et al, IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update, British Journal of Sports Medicine 2018;52:687-697. https://bjsm.bmj.com/content/52/11/687
Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab. 1997;82(2):561-565. doi:10.1210/jcem.82.2.3757 https://pubmed.ncbi.nlm.nih.gov/9024254/
Jørgensen JO, Vahl N, Dall R, Christiansen JS. Resting metabolic rate in healthy adults: relation to growth hormone status and leptin levels. Metabolism. 1998;47(9):1134-1139. doi:10.1016/s0026-0495(98)90289-x https://pubmed.ncbi.nlm.nih.gov/9751244/
Jeon JY, Steadward RD, Wheeler GD, Bell G, McCargar L, Harber V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab. 2003;88(1):402-407. doi:10.1210/jc.2002-020939 https://pubmed.ncbi.nlm.nih.gov/12519883/
Levine JA, Eberhardt NL, Jensen MD. Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. J Clin Endocrinol Metab. 1999;84(8):2751-2754. doi:10.1210/jcem.84.8.5910 https://pubmed.ncbi.nlm.nih.gov/10443673/
Roberts SB, Nicholson M, Staten M, et al. Relationship between circulating leptin and energy expenditure in adult men and women aged 18 years to 81 years. Obes Res. 1997;5(5):459-463. doi:10.1002/j.1550-8528.1997.tb00671.x https://pubmed.ncbi.nlm.nih.gov/9385622/
Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21-34. doi:10.1111/j.1467-789X.2006.00270.x https://pubmed.ncbi.nlm.nih.gov/17212793/
Sinha MK, Opentanova I, Ohannesian JP, et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest. 1996;98(6):1277-1282. doi:10.1172/JCI118913 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC507552/
Lammert O, Grunnet N, Faber P, et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84(2):233-245. https://pubmed.ncbi.nlm.nih.gov/11029975/
Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62(1):19-29. doi:10.1093/ajcn/62.1.19 https://pubmed.ncbi.nlm.nih.gov/7598063/
Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes. 1999;48(2):334-341. doi:10.2337/diabetes.48.2.334 https://pubmed.ncbi.nlm.nih.gov/10334310/
Romon M, Lebel P, Velly C, Marecaux N, Fruchart JC, Dallongeville J. Leptin response to carbohydrate or fat meal and association with subsequent satiety and energy intake. Am J Physiol. 1999;277(5):E855-E861. doi:10.1152/ajpendo.1999.277.5.E855 https://pubmed.ncbi.nlm.nih.gov/10567012/
Kolaczynski JW, Nyce MR, Considine RV, et al. Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro. Diabetes. 1996;45(5):699-701. doi:10.2337/diab.45.5.699 https://pubmed.ncbi.nlm.nih.gov/8621027/
Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762-5771. doi:10.1210/jc.2004-1003 https://pubmed.ncbi.nlm.nih.gov/15531540/
Zarogoulidis, P., Lampaki, S., Turner, J. F., Huang, H., Kakolyris, S., Syrigos, K., & Zarogoulidis, K. (2014). mTOR pathway: A current, up-to-date mini-review (Review). Oncology Letters, 8(6), 2367–2370. http://doi.org/10.3892/ol.2014.2608
Liu, G. Y., & Sabatini, D. M. (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nature Reviews Molecular Cell Biology, 21(4), 183–203. http://doi.org/10.1038/s41580-019-0199-y
Lipton, J. O., & Sahin, M. (2014). The Neurology of mTOR. Neuron, 84(2), 275–291. http://doi.org/10.1016/j.neuron.2014.09.034
Bond, P. (2016). Regulation of mTORC1 by growth factors, energy status, amino acids and mechanical stimuli at a glance. Journal of the International Society of Sports Nutrition, 13(1). http://doi.org/10.1186/s12970-016-0118-y
Adegoke, O. A., Abdullahi, A., & Tavajohi-Fini, P. (2012). mTORC1 and the regulation of skeletal muscle anabolism and mass. Applied Physiology, Nutrition, and Metabolism, 37(3), 395–406. http://doi.org/10.1139/h2012-009
Dibble, C. C., & Manning, B. D. (2013). Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nature Cell Biology, 15(6), 555–564. http://doi.org/10.1038/ncb2763
Mcpherron, A. C., Lawler, A. M., & Lee, S.-J. (1997). Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature, 387(6628), 83–90. http://doi.org/10.1038/387083a0
Armstrong, D. D., & Esser, K. A. (2005). Wnt/β-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. American Journal of Physiology-Cell Physiology, 289(4). http://doi.org/10.1152/ajpcell.00093.2005
Proud, G. C., & Denton, M. R. (1997). Molecular mechanisms for the control of translation by insulin. Biochemical Journal, 328(2), 329–341. http://doi.org/10.1042/bj3280329
Basualto-Alarcón, C., Jorquera, G., Altamirano, F., Jaimovich, E., & Estrada, M. (2013). Testosterone Signals through mTOR and Androgen Receptor to Induce Muscle Hypertrophy. Medicine & Science in Sports & Exercise, 45(9), 1712–1720. http://doi.org/10.1249/mss.0b013e31828cf5f3
Hardie, D. G., Ross, F. A., & Hawley, S. A. (2012). AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology, 13(4), 251–262. http://doi.org/10.1038/nrm3311
Jewell, J. L., & Guan, K.-L. (2013). Nutrient signaling to mTOR and cell growth. Trends in Biochemical Sciences, 38(5), 233–242. http://doi.org/10.1016/j.tibs.2013.01.004
Birk, J. B., & Wojtaszewski, J. F. P. (2006). Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. The Journal of Physiology, 577(3), 1021–1032. http://doi.org/10.1113/jphysiol.2006.120972
Mounier, R., Théret, M., Lantier, L., Foretz, M., & Viollet, B. (2015). Expanding roles for AMPK in skeletal muscle plasticity. Trends in Endocrinology & Metabolism, 26(6), 275–286. http://doi.org/10.1016/j.tem.2015.02.009
Mounier, R., Lantier, L., Leclerc, J., Sotiropoulos, A., Foretz, M., & Viollet, B. (2011). Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle, 10(16), 2640–2646. http://doi.org/10.4161/cc.10.16.17102
Gwinn, D. M., Shackelford, D. B., Egan, D. F., Mihaylova, M. M., Mery, A., Vasquez, D. S., … Shaw, R. J. (2008). AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Molecular Cell, 30(2), 214–226. http://doi.org/10.1016/j.molcel.2008.03.003
Bar-Peled, L., & Sabatini, D. M. (2014). Regulation of mTORC1 by amino acids. Trends in Cell Biology, 24(7), 400–406. http://doi.org/10.1016/j.tcb.2014.03.003
Mohammad A Humayun, Rajavel Elango, Ronald O Ball, Paul B Pencharz, Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique, The American Journal of Clinical Nutrition, Volume 86, Issue 4, October 2007, Pages 995–1002, https://doi.org/10.1093/ajcn/86.4.995
Evaluation of protein requirements for trained strength athletes. M. A. Tarnopolsky, S. A. Atkinson, J. D. MacDougall, A. Chesley, S. Phillips, and H. P. Schwarcz. https://doi.org/10.1152/jappl.1992.73.5.1986
Antonio, J., Peacock, C.A., Ellerbroek, A. et al. The effects of consuming a high protein diet (4.4 g/kg/d) on body composition in resistance-trained individuals. J Int Soc Sports Nutr 11, 19 (2014). https://doi.org/10.1186/1550-2783-11-19
Elango, R., Ball, R.O. & Pencharz, P.B. Amino acid requirements in humans: with a special emphasis on the metabolic availability of amino acids. Amino Acids 37, 19 (2009). https://doi.org/10.1007/s00726-009-0234-y
Marinangeli CPF, House JD. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health [published correction appears in Nutr Rev. 2017 Aug 1;75(8):671]. Nutr Rev. 2017;75(8):658-667. doi:10.1093/nutrit/nux025 https://pubmed.ncbi.nlm.nih.gov/28969364/
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799-806. doi:10.1038/414799a https://pubmed.ncbi.nlm.nih.gov/11742412/
Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev. 2006;7(1):49-58. doi:10.1111/j.1467-789X.2006.00222.x https://pubmed.ncbi.nlm.nih.gov/16436102/
Kanter M. High-Quality Carbohydrates and Physical Performance: Expert Panel Report. Nutr Today. 2018;53(1):35-39. doi:10.1097/NT.0000000000000238 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5794245/
Dong T, Guo M, Zhang P, Sun G, Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: A meta-analysis. PLoS One. 2020;15(1):e0225348. Published 2020 Jan 14. doi:10.1371/journal.pone.0225348 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6959586/
Seidelmann SB, Claggett B, Cheng S, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health. 2018;3(9):e419-e428. doi:10.1016/S2468-2667(18)30135-X https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6339822/
Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5(1):e000354. Published 2017 Feb 23. doi:10.1136/bmjdrc-2016-000354 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5337734/
Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses [published correction appears in Lancet. 2019 Feb 2;393(10170):406]. Lancet. 2019;393(10170):434-445. doi:10.1016/S0140-6736(18)31809-9 https://pubmed.ncbi.nlm.nih.gov/30638909/
Colombani, P.C., Mannhart, C. & Mettler, S. Carbohydrates and exercise performance in non-fasted athletes: A systematic review of studies mimicking real-life. Nutr J 12, 16 (2013). https://doi.org/10.1186/1475-2891-12-16
van Dam, R., Seidell, J. Carbohydrate intake and obesity. Eur J Clin Nutr 61, S75–S99 (2007). https://doi.org/10.1038/sj.ejcn.1602939
Hall KD, Bemis T, Brychta R, et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015;22(3):427-436. doi:10.1016/j.cmet.2015.07.021 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4603544/
Burger KN, Beulens JW, van der Schouw YT, et al. Dietary fiber, carbohydrate quality and quantity, and mortality risk of individuals with diabetes mellitus. PLoS One. 2012;7(8):e43127. doi:10.1371/journal.pone.0043127 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426551/
Karl JP, Roberts SB, Schaefer EJ, et al. Effects of carbohydrate quantity and glycemic index on resting metabolic rate and body composition during weight loss. Obesity (Silver Spring). 2015;23(11):2190-2198. doi:10.1002/oby.21268 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4634125/
Chambers ES, Byrne CS, Frost G. Carbohydrate and human health: is it all about quality?. Lancet. 2019;393(10170):384-386. doi:10.1016/S0140-6736(18)32468-1 https://pubmed.ncbi.nlm.nih.gov/30638908/
Gaesser GA. Carbohydrate quantity and quality in relation to body mass index. J Am Diet Assoc. 2007;107(10):1768-1780. doi:10.1016/j.jada.2007.07.011 https://pubmed.ncbi.nlm.nih.gov/17904937/
van Dam RM, Seidell JC. Carbohydrate intake and obesity. Eur J Clin Nutr. 2007;61 Suppl 1:S75-S99. doi:10.1038/sj.ejcn.1602939 https://pubmed.ncbi.nlm.nih.gov/17992188/
Wylie-Rosett J, Segal-Isaacson CJ, Segal-Isaacson A. Carbohydrates and increases in obesity: does the type of carbohydrate make a difference?. Obes Res. 2004;12 Suppl 2:124S-9S. doi:10.1038/oby.2004.277 https://pubmed.ncbi.nlm.nih.gov/15601960/
Zhang X, Yang S, Chen J, Su Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front Endocrinol (Lausanne). 2019;9:802. Published 2019 Jan 24. doi:10.3389/fendo.2018.00802 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6353800/
Melkonian EA, Asuka E, Schury MP. Physiology, Gluconeogenesis. [Updated 2021 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541119/
Chourpiliadis C, Mohiuddin SS. Biochemistry, Gluconeogenesis. [Updated 2021 Aug 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544346/
Schutz Y. Protein turnover, ureagenesis and gluconeogenesis. Int J Vitam Nutr Res. 2011;81(2-3):101-107. doi:10.1024/0300-9831/a000064 https://pubmed.ncbi.nlm.nih.gov/22139560/
Veldhorst MA, Westerterp-Plantenga MS, Westerterp KR. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am J Clin Nutr. 2009;90(3):519-526. doi:10.3945/ajcn.2009.27834 https://pubmed.ncbi.nlm.nih.gov/19640952/
Gardner CD, Offringa LC, Hartle JC, Kapphahn K, Cherin R. Weight loss on low-fat vs. low-carbohydrate diets by insulin resistance status among overweight adults and adults with obesity: A randomized pilot trial. Obesity (Silver Spring). 2016;24(1):79-86. doi:10.1002/oby.21331 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5898445/
Astrup A, Hjorth MF. Low-Fat or Low Carb for Weight Loss? It Depends on Your Glucose Metabolism. EBioMedicine. 2017;22:20-21. doi:10.1016/j.ebiom.2017.07.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5672079/
Åberg S, Mann J, Neumann S, Ross AB, Reynolds AN. Whole-Grain Processing and Glycemic Control in Type 2 Diabetes: A Randomized Crossover Trial. Diabetes Care. 2020;43(8):1717-1723. doi:10.2337/dc20-0263 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7372063/
Clark CM Jr. Glycemic control and hypoglycemia: is the loser the winner? Response to Perlmuter et al. Diabetes Care. 2009;32(3):e32-e33. doi:10.2337/dc08-2047 https://pubmed.ncbi.nlm.nih.gov/19246583/
Hardy DS, Garvin JT, Xu H. Carbohydrate quality, glycemic index, glycemic load and cardiometabolic risks in the US, Europe and Asia: A dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2020;30(6):853-871. doi:10.1016/j.numecd.2019.12.050 https://pubmed.ncbi.nlm.nih.gov/32278608/
Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr. 2007;61 Suppl 1:S122-S131. doi:10.1038/sj.ejcn.1602942 https://pubmed.ncbi.nlm.nih.gov/17992183/
Bao J, de Jong V, Atkinson F, Petocz P, Brand-Miller JC. Food insulin index: physiologic basis for predicting insulin demand evoked by composite meals. Am J Clin Nutr. 2009;90(4):986-992. doi:10.3945/ajcn.2009.27720 https://pubmed.ncbi.nlm.nih.gov/19710196/
Zeevi D, Korem T, Zmora N, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163(5):1079-1094. doi:10.1016/j.cell.2015.11.001 https://pubmed.ncbi.nlm.nih.gov/26590418/
Gertsch J. The Metabolic Plant Feedback Hypothesis: How Plant Secondary Metabolites Nonspecifically Impact Human Health. Planta Med. 2016;82(11-12):920-929. doi:10.1055/s-0042-108340 https://pubmed.ncbi.nlm.nih.gov/27286339/
Kim, Y., & Je, Y. (2016). Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Archives of Cardiovascular Diseases, 109(1), 39–54. http://doi.org/10.1016/j.acvd.2015.09.005.
Veronese, N., Solmi, M., Caruso, M. G., Giannelli, G., Osella, A. R., Evangelou, E., … Tzoulaki, I. (2018). Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. The American Journal of Clinical Nutrition, 107(3), 436–444. http://doi.org/10.1093/ajcn/nqx082
Dietary reference intakes (DRIs). Institute of Medicine. https://www.nap.edu/catalog/11537/dietary-reference-intakes-the-essential-guide-to-nutrient-requirements
2015-2020 Dietary Guidelines for Americans. https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/
Holscher, H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes, 8(2), 172–184. http://doi.org/10.1080/19490976.2017.1290756
Rowland, I., Gibson, G., Heinken, A., Scott, K., Swann, J., Thiele, I., & Tuohy, K. (2017). Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition, 57(1), 1–24. http://doi.org/10.1007/s00394-017-1445-8
Conlon, M., & Bird, A. (2014). The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients, 7(1), 17–44. http://doi.org/10.3390/nu7010017
Murray, K., Wilkinson-Smith, V., Hoad, C., Costigan, C., Cox, E., Lam, C., … Spiller, R. C. (2014). Differential Effects of FODMAPs (Fermentable Oligo-, Di-, Mono-Saccharides and Polyols) on Small and Large Intestinal Contents in Healthy Subjects Shown by MRI. American Journal of Gastroenterology, 109(1), 110–119. http://doi.org/10.1038/ajg.2013.386
Maruvada, P., Leone, V., Kaplan, L. M., & Chang, E. B. (2017). The Human Microbiome and Obesity: Moving beyond Associations. Cell Host & Microbe, 22(5), 589–599. http://doi.org/10.1016/j.chom.2017.10.005
Li, Z.-H., Zhong, W.-F., Liu, S., Kraus, V. B., Zhang, Y.-J., Gao, X., … Mao, C. (2020). Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: evidence from a large population based cohort study. Bmj, m456. http://doi.org/10.1136/bmj.m456
Gavino, V. C., & Gavino, G. R. (1992). Adipose hormone-sensitive lipase preferentially releases polyunsaturated fatty acids from triglycerides. Lipids, 27(12), 950–954. http://doi.org/10.1007/bf02535570
Di Pasquale MG. The essentials of essential fatty acids. J Diet Suppl. 2009;6(2):143-161. doi:10.1080/19390210902861841 https://pubmed.ncbi.nlm.nih.gov/22435414/
Das UN. Essential Fatty acids – a review. Curr Pharm Biotechnol. 2006;7(6):467-482. doi:10.2174/138920106779116856 https://pubmed.ncbi.nlm.nih.gov/17168664/
Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods- a review. J Food Sci Technol. 2014;51(10):2289-2303. doi:10.1007/s13197-012-0677-0 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4190204/
Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int J Mol Sci. 2017;18(12):2645. Published 2017 Dec 7. doi:10.3390/ijms18122645 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5751248/
Wene JD, Connor WE, DenBesten L. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. The Journal of Clinical Investigation. 1975 Jul;56(1):127-134. DOI: 10.1172/jci108061. PMID: 806609; PMCID: PMC436563. https://europepmc.org/article/PMC/436563
Wainwright P.E. (1997) Essential Fatty Acids and Behavior. In: Yehuda S., Mostofsky D.I. (eds) Handbook of Essential Fatty Acid Biology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-4757-2582-7_14
Holman R.T. (1997) ω3 and ω6 Essential Fatty Acid Status in Human Health and Disease. In: Yehuda S., Mostofsky D.I. (eds) Handbook of Essential Fatty Acid Biology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-4757-2582-7_7
Holman R.T. (1977) Essential Fatty Acids in Human Nutrition. In: Bazán N.G., Brenner R.R., Giusto N.M. (eds) Function and Biosynthesis of Lipids. Advances in Experimental Medicine and Biology, vol 83. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-3276-3_48
Tang M , Liu Y , Wang L , et al. An Ω-3 fatty acid-deficient diet during gestation induces depressive-like behavior in rats: the role of the hypothalamo-pituitary-adrenal (HPA) system. Food Funct. 2018;9(6):3481-3488. doi:10.1039/c7fo01714f https://pubmed.ncbi.nlm.nih.gov/29882567/
Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7(7):CD003177. Published 2018 Jul 18. doi:10.1002/14651858.CD003177.pub3 https://pubmed.ncbi.nlm.nih.gov/30019766/
Larrieu T, Layé S. Food for Mood: Relevance of Nutritional Omega-3 Fatty Acids for Depression and Anxiety. Front Physiol. 2018;9:1047. Published 2018 Aug 6. doi:10.3389/fphys.2018.01047 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6087749/
https://www.nccih.nih.gov/health/omega3-supplements-in-depth
Meyer, B.J., Mann, N.J., Lewis, J.L. et al. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38, 391–398 (2003). https://doi.org/10.1007/s11745-003-1074-0
McDonald, C., Bauer, J., Capra, S. et al. The muscle mass, omega-3, diet, exercise and lifestyle (MODEL) study – a randomised controlled trial for women who have completed breast cancer treatment. BMC Cancer 14, 264 (2014). https://doi.org/10.1186/1471-2407-14-264
Leckey JJ, Hoffman NJ, Parr EB, et al. High dietary fat intake increases fat oxidation and reduces skeletal muscle mitochondrial respiration in trained humans. FASEB J. 2018;32(6):2979-2991. doi:10.1096/fj.201700993R https://pubmed.ncbi.nlm.nih.gov/29401600/
Liu AG, Ford NA, Hu FB, Zelman KM, Mozaffarian D, Kris-Etherton PM. A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutr J. 2017;16(1):53. Published 2017 Aug 30. doi:10.1186/s12937-017-0271-4 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5577766/
Zhu Y, Bo Y, Liu Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: a dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019;18(1):91. Published 2019 Apr 6. doi:10.1186/s12944-019-1035-2 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6451787/
Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73(6):1019-1026. doi:10.1093/ajcn/73.6.1019 https://pubmed.ncbi.nlm.nih.gov/11382654/
Dong J, Beard JD, Umbach DM, et al. Dietary fat intake and risk for Parkinson’s disease. Mov Disord. 2014;29(13):1623-1630. doi:10.1002/mds.26032 https://pubmed.ncbi.nlm.nih.gov/25186946/
Han J, Jiang Y, Liu X, et al. Dietary Fat Intake and Risk of Gastric Cancer: A Meta-Analysis of Observational Studies. PLoS One. 2015;10(9):e0138580. Published 2015 Sep 24. doi:10.1371/journal.pone.0138580 https://pubmed.ncbi.nlm.nih.gov/26402223/
Lowery LM. Dietary fat and sports nutrition: a primer. J Sports Sci Med. 2004;3(3):106-117. Published 2004 Sep 1. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3905293/
Helms ER, Aragon AA, Fitschen PJ. Evidence-based recommendations for natural bodybuilding contest preparation: nutrition and supplementation. J Int Soc Sports Nutr. 2014;11:20. Published 2014 May 12. doi:10.1186/1550-2783-11-20 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4033492/
Pahwa R, Jialal I. Atherosclerosis. [Updated 2021 Sep 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507799/
Linton MRF, Yancey PG, Davies SS, et al. The Role of Lipids and Lipoproteins in Atherosclerosis. [Updated 2019 Jan 3]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343489/
Heileson JL. Dietary saturated fat and heart disease: a narrative review. Nutr Rev. 2020;78(6):474-485. doi:10.1093/nutrit/nuz091 https://pubmed.ncbi.nlm.nih.gov/31841151/
Clifton PM, Keogh JB. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr Metab Cardiovasc Dis. 2017;27(12):1060-1080. doi:10.1016/j.numecd.2017.10.010 https://pubmed.ncbi.nlm.nih.gov/29174025/
Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;8(8):CD011737. Published 2020 Aug 21. doi:10.1002/14651858.CD011737.pub3 https://pubmed.ncbi.nlm.nih.gov/32827219/
Nettleton JA, Brouwer IA, Geleijnse JM, Hornstra G. Saturated Fat Consumption and Risk of Coronary Heart Disease and Ischemic Stroke: A Science Update. Ann Nutr Metab. 2017;70(1):26-33. doi:10.1159/000455681 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5475232/
Houston M. The relationship of saturated fats and coronary heart disease: fa(c)t or fiction? A commentary. Ther Adv Cardiovasc Dis. 2018;12(2):33-37. doi:10.1177/1753944717742549 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5933589/
Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep. 2010;12(6):384-390. doi:10.1007/s11883-010-0131-6 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2943062/
Temple NJ. Fat, Sugar, Whole Grains and Heart Disease: 50 Years of Confusion. Nutrients. 2018;10(1):39. Published 2018 Jan 4. doi:10.3390/nu10010039 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5793267/
Linton MRF, Yancey PG, Davies SS, et al. The Role of Lipids and Lipoproteins in Atherosclerosis. [Updated 2019 Jan 3]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343489/
Törnwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK. Alpha-tocopherol (vitamin E) and beta-carotene supplementation does not affect the risk for large abdominal aortic aneurysm in a controlled trial. Atherosclerosis. 2001;157(1):167-173. doi:10.1016/s0021-9150(00)00694-8 https://pubmed.ncbi.nlm.nih.gov/11427217/
Kinlay S, Behrendt D, Fang JC, et al. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol. 2004;43(4):629-634. doi:10.1016/j.jacc.2003.08.051 https://pubmed.ncbi.nlm.nih.gov/14975474/
Devaraj S, Tang R, Adams-Huet B, et al. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr. 2007;86(5):1392-1398. doi:10.1093/ajcn/86.5.1392 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2692902/
Erkki Vartiainen, Tiina Laatikainen, Markku Peltonen, Anne Juolevi, Satu Männistö, Jouko Sundvall, Pekka Jousilahti, Veikko Salomaa, Liisa Valsta, Pekka Puska, Thirty-five-year trends in cardiovascular risk factors in Finland, International Journal of Epidemiology, Volume 39, Issue 2, April 2010, Pages 504–518, https://doi.org/10.1093/ije/dyp330
Penny M Kris-Etherton, Thomas A Pearson, Ying Wan, Rebecca L Hargrove, Kristin Moriarty, Valerie Fishell, Terry D Etherton, High–monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations, The American Journal of Clinical Nutrition, Volume 70, Issue 6, December 1999, Pages 1009–1015, https://doi.org/10.1093/ajcn/70.6.1009
Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112(7):1029-1041.e10415. doi:10.1016/j.jand.2012.03.029 https://pubmed.ncbi.nlm.nih.gov/22889633/
Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674-688. doi:10.3181/0711-MR-311 https://pubmed.ncbi.nlm.nih.gov/18408140/
Jeromson S, Gallagher IJ, Galloway SD, Hamilton DL. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar Drugs. 2015;13(11):6977-7004. Published 2015 Nov 19. doi:10.3390/md13116977 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4663562/
James P DeLany, Marlene M Windhauser, Catherine M Champagne, George A Bray, Differential oxidation of individual dietary fatty acids in humans, The American Journal of Clinical Nutrition, Volume 72, Issue 4, October 2000, Pages 905–911, https://doi.org/10.1093/ajcn/72.4.905
Markworth JF, Cameron-Smith D. Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. Am J Physiol Cell Physiol. 2013;304(1):C56-C67. doi:10.1152/ajpcell.00038.2012 https://pubmed.ncbi.nlm.nih.gov/23076795/
Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3(1):1-7. doi:10.3945/an.111.000893 https://pubmed.ncbi.nlm.nih.gov/22332096/
Nichols PD, McManus A, Krail K, Sinclair AJ, Miller M. Recent advances in omega-3: Health Benefits, Sources, Products and Bioavailability. Nutrients. 2014;6(9):3727-3733. Published 2014 Sep 16. doi:10.3390/nu6093727 https://pubmed.ncbi.nlm.nih.gov/25255830/
Calder PC, Yaqoob P. Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors. 2009;35(3):266-272. doi:10.1002/biof.42 https://pubmed.ncbi.nlm.nih.gov/19391122/
Shahidi F, Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol. 2018;9:345-381. doi:10.1146/annurev-food-111317-095850 https://pubmed.ncbi.nlm.nih.gov/29350557/
Gammone MA, Riccioni G, Parrinello G, D’Orazio N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients. 2018;11(1):46. Published 2018 Dec 27. doi:10.3390/nu11010046 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6357022/
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. Published 2015 Apr 21. doi:10.3389/fnagi.2015.00052 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4404917/
Mohebi-Nejad A, Bikdeli B. Omega-3 supplements and cardiovascular diseases. Tanaffos. 2014;13(1):6-14. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4153275/
Ander BP, Dupasquier CM, Prociuk MA, Pierce GN. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp Clin Cardiol. 2003;8(4):164-172. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2719153/
Da Boit M, Hunter AM, Gray SR. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism. 2017;66:45-54. doi:10.1016/j.metabol.2016.10.007 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5155640/
Imamura F, Micha R, Wu JH, et al. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016;13(7):e1002087. Published 2016 Jul 19. doi:10.1371/journal.pmed.1002087 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4951141/
Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. Published 2010 Mar 23. doi:10.1371/journal.pmed.1000252 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2843598/
Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J. 2017;16(1):30. Published 2017 May 19. doi:10.1186/s12937-017-0254-5 https://pubmed.ncbi.nlm.nih.gov/28526025/
Ginter E, Simko V. New data on harmful effects of trans-fatty acids. Bratisl Lek Listy. 2016;117(5):251-253. doi:10.4149/bll_2016_048 https://pubmed.ncbi.nlm.nih.gov/27215959/
Ganguly R, Pierce GN. The toxicity of dietary trans fats. Food Chem Toxicol. 2015;78:170-176. doi:10.1016/j.fct.2015.02.004 https://pubmed.ncbi.nlm.nih.gov/25684416/
Dhaka V, Gulia N, Ahlawat KS, Khatkar BS. Trans fats-sources, health risks and alternative approach – A review. J Food Sci Technol. 2011;48(5):534-541. doi:10.1007/s13197-010-0225-8 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3551118/
Penny M. Kris-Etherton (2010) Trans-Fats and Coronary Heart Disease, Critical Reviews in Food Science and Nutrition, 50:sup1, 29-30, DOI: 10.1080/10408398.2010.526872
Schonfeld G, Patsch W, Rudel LL, Nelson C, Epstein M, Olson RE. Effects of dietary cholesterol and fatty acids on plasma lipoproteins. J Clin Invest. 1982;69(5):1072-1080. doi:10.1172/jci110542 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC370171/
Fielding CJ, Havel RJ, Todd KM, et al. Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young men. J Clin Invest. 1995;95(2):611-618. doi:10.1172/JCI117705 https://pubmed.ncbi.nlm.nih.gov/7860745/
Leiper, J. (1998). Intestinal Water Absorption – Implications for the Formulation of Rehydration Solutions. International Journal of Sports Medicine, 19(S 2). http://doi.org/10.1055/s-2007-971977
Newburgh, L. H., Johnston, M. W., & Falcon-Lesses, M. (1930). Measurement Of Total Water Exchange 1. Journal of Clinical Investigation, 8(2), 161–196. http://doi.org/10.1172/jci100259
Howard G, Bartram J. Domestic Water Quantity, Service, Level and Health. World Health Organization, 2003. Ref Type: Report
Food and Nutrition Board. Recommended Daily Allowances. 10 ed. Washington, DC: National Academy Press, 1989.
Food and Nutrition Board. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academies Press, 2004.
Scott JM, Linderman JR, Deuster PA. Hydration: Tactical and Practical Strategies [published online ahead of print, 2023 Feb 24]. J Spec Oper Med. 2023;QOBG-HTOX. doi:10.55460/QOBG-HTOX https://doi.org/10.55460/qobg-htox
Grandjean, A. C., Reimers, K. J., Bannick, K. E., & Haven, M. C. (2000). The Effect of Caffeinated, Non-Caffeinated, Caloric and Non-Caloric Beverages on Hydration. Journal of the American College of Nutrition, 19(5), 591–600. http://doi.org/10.1080/07315724.2000.10718956
Montain, S. J., Latzka, W. A., & Sawka, M. N. (1999). Fluid Replacement Recommendations for Training in Hot Weather. Military Medicine, 164(7), 502–508. http://doi.org/10.1093/milmed/164.7.502
Epstein, Y., & Armstrong, L. E. (1999). Fluid-Electrolyte Balance during Labor and Exercise: Concepts and Misconceptions. International Journal of Sport Nutrition, 9(1), 1–12. http://doi.org/10.1123/ijsn.9.1.1
Latzka, W. A., & Montain, S. J. (1999). Water And Electrolyte Requirements For Exercise. Clinics in Sports Medicine, 18(3), 513–524. http://doi.org/10.1016/s0278-5919(05)70165-4
Helms, E.R., Aragon, A.A. & Fitschen, P.J. Evidence-based recommendations for natural bodybuilding contest preparation: nutrition and supplementation. J Int Soc Sports Nutr 11, 20 (2014). https://doi.org/10.1186/1550-2783-11-20
Kleiner SM, Bazzarre TL, Litchford MD. Metabolic profiles, diet, and health practices of championship male and female bodybuilders. J Am Diet Assoc. 1990;90(7):962-967. https://pubmed.ncbi.nlm.nih.gov/2365938/
Lambert CP, Frank LL, Evans WJ. Macronutrient considerations for the sport of bodybuilding. Sports Med. 2004;34(5):317-327. doi:10.2165/00007256-200434050-00004 https://pubmed.ncbi.nlm.nih.gov/15107010/
Hall, K. What is the required energy deficit per unit weight loss?. Int J Obes 32, 573–576 (2008). https://doi.org/10.1038/sj.ijo.0803720
Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis [published correction appears in Am J Clin Nutr. 2014 Nov;100(5):1405]. Am J Clin Nutr. 2013;97(5):990-994. doi:10.3945/ajcn.112.050310 https://pubmed.ncbi.nlm.nih.gov/23535105/
Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass [published correction appears in J Clin Endocrinol Metab. 2016 May;101(5):2266]. J Clin Endocrinol Metab. 2012;97(7):2489-2496. doi:10.1210/jc.2012-1444 https://pubmed.ncbi.nlm.nih.gov/22535969/
Mero AA, Huovinen H, Matintupa O, et al. Moderate energy restriction with high protein diet results in healthier outcome in women. J Int Soc Sports Nutr. 2010;7(1):4. Published 2010 Jan 25. doi:10.1186/1550-2783-7-4 https://pubmed.ncbi.nlm.nih.gov/20205751/
Phillips SM, Van Loon LJ. Dietary protein for athletes: from requirements to optimum adaptation. J Sports Sci. 2011;29 Suppl 1:S29-S38. doi:10.1080/02640414.2011.619204 https://pubmed.ncbi.nlm.nih.gov/22150425/
Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010;42(2):326-337. doi:10.1249/MSS.0b013e3181b2ef8e https://pubmed.ncbi.nlm.nih.gov/19927027/
Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36(3):239-262. doi:10.2165/00007256-200636030-00005 https://pubmed.ncbi.nlm.nih.gov/16526835/
Pendergast DR, Leddy JJ, Venkatraman JT. A perspective on fat intake in athletes. J Am Coll Nutr. 2000;19(3):345-350. doi:10.1080/07315724.2000.10718930 https://pubmed.ncbi.nlm.nih.gov/10872896/
Turocy PS, DePalma BF, Horswill CA, et al. National Athletic Trainers’ Association position statement: safe weight loss and maintenance practices in sport and exercise. J Athl Train. 2011;46(3):322-336. doi:10.4085/1062-6050-46.3.322 https://pubmed.ncbi.nlm.nih.gov/21669104/
Helms ER, Prnjak K, Linardon J. Towards a Sustainable Nutrition Paradigm in Physique Sport: A Narrative Review. Sports (Basel). 2019;7(7):172. Published 2019 Jul 16. doi:10.3390/sports7070172 https://pubmed.ncbi.nlm.nih.gov/31315180/
Iraki J, Fitschen P, Espinar S, Helms E. Nutrition Recommendations for Bodybuilders in the Off-Season: A Narrative Review. Sports (Basel). 2019;7(7):154. Published 2019 Jun 26. doi:10.3390/sports7070154 https://pubmed.ncbi.nlm.nih.gov/31247944/

