What actually controls when you fall asleep? What determines when you feel sleepy, when you wake up, and how well you sleep once you drift off? If you are like most people, you have probably never thought much about this, however, if you are looking to improve your sleep, understanding sleep regulation is crucial. Once you grasp what’s driving your sleep-wake cycle, you can begin to work intelligently with these systems rather than fighting against them.
Sleep regulation isn’t mysterious or arbitrary. Two main processes govern when you sleep and how well you sleep: your circadian rhythm (your internal biological clock synced to the day-night cycle) and sleep-wake homeostasis (the pressure to sleep that builds the longer you’re awake). These systems can work in harmony, producing effortless sleep and natural wakefulness, or they can fall out of alignment, leaving you tired during the day but wired at night. Most sleep problems stem from a misalignment between these regulatory systems and your actual behaviour.
Before diving into how these systems work, we need to briefly familiarise ourselves with the relevant brain structures. The brain isn’t a homogenous unit, and it’s composed of distinct regions with specialised functions, and several of these regions orchestrate your sleep-wake cycle.
Table of Contents
- 1 The Brain’s Sleep-Wake Control Centre
- 2 Circadian Rhythms: Your Internal Biological Clock
- 3 Sleep-Wake Homeostasis: The Pressure to Sleep
- 4 The Two-Process Model: When Systems Align or Conflict
- 5 Circadian Rhythm Disorders: When Your Clock Won’t Sync
- 6 Chronotypes: Real Variation or Poor Sleep Hygiene?
- 7 Age, Sex, and Individual Variation in Sleep Regulation
- 8 Environmental and Behavioural Disruption in Modern Life
- 9 Working With Your Biology, Not Against It
- 10 Sleep Regulation Conclusion: Two Systems, One Goal
- 11 Author
The Brain’s Sleep-Wake Control Centre
The hypothalamus sits at the centre of sleep regulation. This small but crucial brain region controls numerous vital functions, including body temperature, hunger, hormone release, and yes, sleep. Nestled within the hypothalamus is the suprachiasmatic nucleus (SCN), often called the master clock. The SCN sits directly above the optic chiasm (where the optic nerves from your eyes meet the brain) and this positioning is no accident. The SCN receives direct input from your eyes about environmental light, allowing it to sync your internal biological rhythms to the external day-night cycle.
The SCN doesn’t work alone. It communicates with the pineal gland, a small gland nestled between the brain’s two hemispheres. In response to signals from the SCN indicating darkness, the pineal gland secretes melatonin, which is a hormone that signals to your body and brain that it’s time to sleep. This communication pathway between the SCN and pineal gland is central to how the daily light-dark cycle influences your sleep.
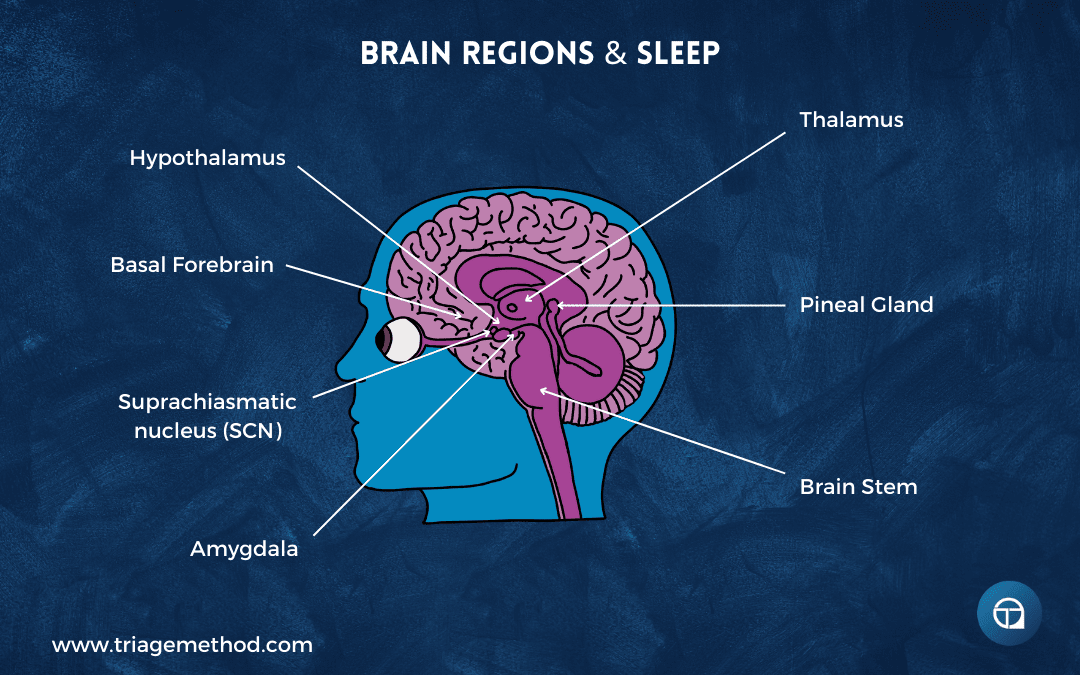
The brain stem also plays a vital role in sleep regulation. It receives instructions from the hypothalamus and produces neurotransmitters like GABA (gamma-aminobutyric acid) that reduce wakefulness, or more accurately, dampen arousal, throughout the brain. The brain stem is also responsible for the muscle relaxation that occurs during REM sleep, which is why you don’t physically act out your dreams and why sleep paralysis can occur if you wake before this inhibition lifts.
The thalamus serves as a sensory gatekeeper. During waking hours, it relays information from your senses to the cerebral cortex, allowing you to process the world around you. During most of sleep, the thalamus goes quiet, cutting off this stream of sensory information so your cortex can perform its restorative work (including memory consolidation). During REM sleep, however, the thalamus reactivates, which is why dreams include sights, sounds, and other sensations, and why your alarm can sometimes become incorporated into a dream if it goes off during REM.
The basal forebrain and midbrain are involved in wakefulness and the release of acetylcholine, a neurotransmitter that promotes arousal. These regions respond to adenosine levels, which is a critical player in sleep-wake homeostasis and sleep regulation, that we’ll explore shortly. The amygdala, primarily known for processing emotions, becomes particularly active during REM sleep, playing a role in consolidating emotional memories and experiences.
These brain regions work together in a coordinated fashion, with wake-promoting systems (involving neurotransmitters like orexin, histamine, norepinephrine, serotonin, and dopamine) and sleep-promoting systems (involving GABA, adenosine, and melatonin) acting like a flip-flop switch, where when one system is on, the other is off. This arrangement helps prevent you from being stuck in an intermediate state between sleep and wakefulness, though when this switch doesn’t operate cleanly, you can experience that uncomfortable “tired but wired” feeling.
With this neurological foundation in place, we can now explore the two main mechanisms of sleep regulation.
Circadian Rhythms: Your Internal Biological Clock
The term “circadian” derives from the Latin words “circa” (around) and “diem” (day); literally meaning “around the day.” Circadian rhythms refer to the roughly 24-hour cycles that govern numerous biological processes. Most obviously, you’re typically awake during daylight hours and asleep at night. This isn’t arbitrary, it’s because we evolved to become entrained to the solar cycle, using external signals to set our internal body clocks.
Now, your body doesn’t have a single clock that determines things, but rather a network of clocks throughout your tissues and organs. These are peripheral clocks that control local functions. Your liver has a clock that regulates metabolic processes. Your muscles have clocks influencing performance capacity across the day. Your digestive system has clocks coordinating enzyme secretion and nutrient absorption. Nearly every organ system operates on circadian schedules.
But these peripheral clocks don’t operate independently. They all synchronise to the master clock in the SCN, which is why understanding what regulates the SCN is so crucial to understanding sleep regulation. The SCN receives environmental time cues (primarily light, but also temperature and other signals) and then broadcasts timing information throughout the body via neural signals and hormonal messengers like melatonin and cortisol.
The peripheral clocks receive these signals and adjust their timing accordingly. This hierarchical system ensures coordination. Your digestive clock expects food during waking hours when your master clock indicates daytime, while your liver’s metabolic clock prepares for different functions during the fasting period when your master clock signals nighttime sleep.
However, peripheral clocks can be pulled out of sync with the master clock by strong local signals. Eating at unusual times, for instance, can shift digestive system clocks even while your SCN maintains its schedule based on light. Temperature changes can affect peripheral clocks in muscles and other tissues. This desynchronisation between master and peripheral clocks contributes to the malaise you feel with jet lag or shift work. It’s not just that your sleep timing is off, but that your internal orchestra is playing different songs simultaneously.
What is interesting here is that your internal master clock doesn’t run on exactly 24 hours. For most people, the natural period is closer to 24.2 hours. Close, but not perfect. Some individuals run slightly longer, others slightly shorter, and this contributes to natural chronotype variation. Without environmental cues to reset it daily, your internal clock would gradually drift out of sync with the actual day-night cycle, like a watch that runs a few minutes fast. Within a few weeks, you’d be completely out of phase with the external world.
This is why environmental signals (called zeitgebers, which is German for “time-givers”) are essential. These zeitgebers constantly nudge your internal clock back into alignment with the 24-hour day, correcting that slight drift. The SCN is continuously making small adjustments based on these external cues, keeping your biology synced to the environment.
Your body need to track time so precisely because different physiological processes need to occur at specific times and in particular sequences. Your body releases cortisol and other hormones to wake you in the morning. Body temperature rises during the day and falls at night. Blood pressure follows a daily pattern, typically dipping during sleep. Metabolism shifts between different modes: daytime feeding and activity versus nighttime repair and fasting. Immune function varies across the day, with certain immune responses stronger at specific times. Cell division and DNA repair are timed to occur when you’re least likely to encounter UV radiation.
If the systems meant to wake you up were activated while you were trying to sleep, you’d find it extremely difficult to drift off. If digestive enzymes were secreted when no food was expected, you’d waste metabolic resources. If immune responses peaked at the wrong times, you’d be more vulnerable to infection. Proper timing isn’t just convenient; it’s essential for health and survival.
Circadian rhythms govern far more than just sleep. Nearly every system in your body operates on a circadian schedule. Understanding what influences these rhythms gives you leverage over your sleep-wake cycle and, more broadly, over your daily functioning and long-term health.
Light: The Primary Zeitgeber
If you had to estimate the time using only environmental cues, you’d probably look at the sun’s position in the sky. When the sun is high, it’s midday. When it’s low, it’s either morning or evening. If you knew what hemisphere of the planet you are on, you would be able to tell if it was morning or evening, or alternatively, if you followed the movement of the sun over time, you would see if it was going up or going down (and thus you would know if it was morning or evening). Your body uses essentially the same strategy.
Light is the most powerful zeitgeber for your circadian system, and your SCN uses light information from your eyes to set the time. But this isn’t a simple on-off switch responding to the presence or absence of light. Instead, your SCN analyses the specific wavelength composition and intensity of light entering your eyes, sophisticatedly extracting information about the time of day.
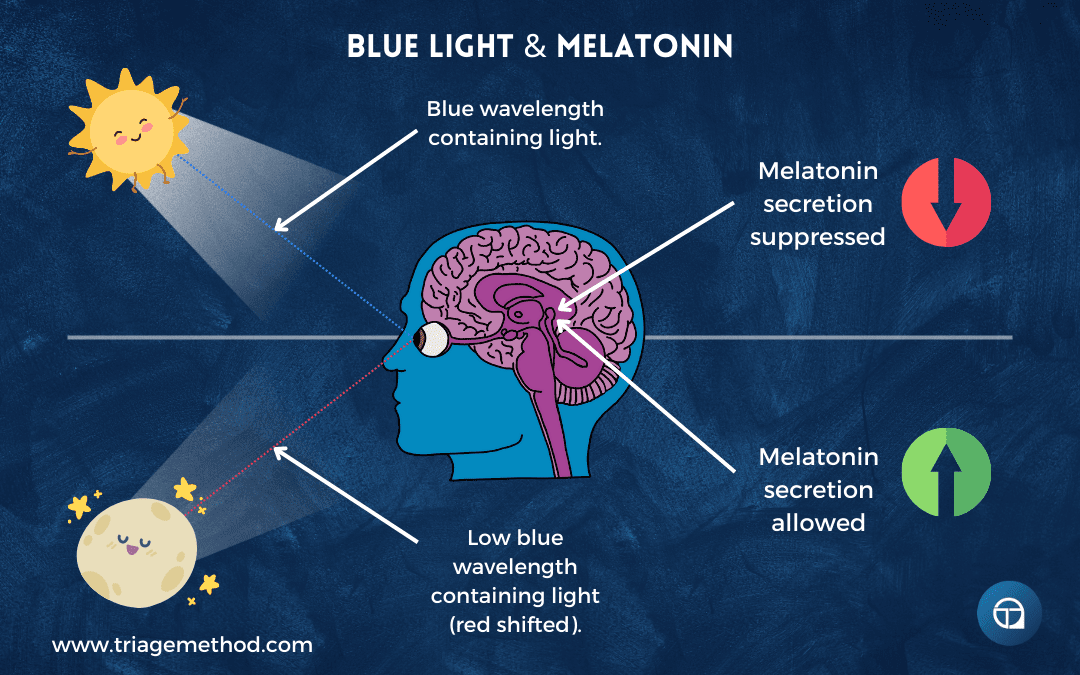
You see, sunlight changes its properties throughout the day based on the sun’s angle and how light travels through the atmosphere. During the middle of the day, when the sun is high in the sky, sunlight travels through less atmosphere to reach you. This light is intense (often 100,000 lux or more on a clear day) and rich in blue wavelengths (around 480 nanometres). Early morning and late evening light is less intense, perhaps 1,000-10,000 lux, and shifts toward red wavelengths as sunlight travels through more atmosphere at oblique angles, scattering shorter blue wavelengths and allowing longer red wavelengths through, which is why you see those dramatic red and orange sunrises and sunsets.
Your eyes contain specialised cells called intrinsically photosensitive retinal ganglion cells (ipRGCs) that are distinct from the rods and cones you use for vision. These ipRGCs contain melanopsin, a photopigment particularly sensitive to blue light around 480 nanometres. Whilst rods and cones send signals to visual processing areas of your brain so you can see, ipRGCs send signals directly to the SCN along a dedicated pathway called the retinohypothalamic tract. This means your circadian system is receiving light information independently of your conscious visual experience.
The SCN interprets the spectral composition, intensity, and timing of light to determine what time of day it is. Blue-rich, intense light signals daytime. Red-shifted, dimmer light signals dawn or dusk. Darkness signals night. The duration of light exposure matters too, as your circadian system integrates light information over time, so sustained exposure to dim light can have similar effects to brief exposure to bright light, though bright light is generally more potent for shifting circadian phase.
Individual differences exist in light sensitivity. Some people’s circadian systems are more sensitive to light, shifting phase more readily in response to light exposure. Others are less sensitive, requiring stronger light signals to shift their clocks. This variation likely contributes to individual differences in chronotype and in vulnerability to circadian disruption from artificial light at night. Age also affects light sensitivity, and children and adolescents appear more sensitive to evening light than adults, which may partly explain why teenagers struggle with early schedules, and elderly individuals often show reduced circadian responses to light, potentially contributing to their sleep difficulties.
When the SCN receives signals indicating darkness or dim, red-shifted light, it instructs the pineal gland to secrete melatonin. When it receives signals indicating bright, blue-rich light, melatonin secretion is suppressed. This light-dependent melatonin secretion is fundamental to sleep regulation.
It’s worth noting that whilst the eyes are by far the primary route for light information to reach the SCN, you do have melanopsin-containing cells in other parts of your body, such as the skin, blood vessels, and even some internal tissues. These peripheral photoreceptors may play roles in local circadian regulation, such as blood vessel dilation in response to light on the skin, and might contribute subtle inputs to overall circadian timing. However, they’re far weaker signals than the light entering your eyes. In individuals with visual impairments or blindness, these peripheral sensors might play larger roles, though people who are completely blind often struggle with circadian rhythm disorders because they lack the dominant light signal entirely.
Understanding this system has profound practical implications, which is why light management forms a cornerstone of sleep hygiene. But first, let’s clarify what melatonin actually does.
Melatonin: The Timing Signal, Not a Sleeping Pill
Melatonin is widely misunderstood. It’s often thought of as a sleeping pill and something that directly induces sleep. This isn’t accurate. Melatonin is better understood as a timing signal, a chemical message that tells your body and brain, “It’s nighttime. Sleep should happen soon.”
The dim light melatonin onset (DLMO) (the time when melatonin begins rising in the evening) typically occurs about two hours before your habitual bedtime. This rise is triggered when light levels drop below about 200 lux, and particularly when blue wavelengths diminish. Melatonin levels continue rising through the night, typically peak in the early morning hours around 2-4 am, then decline as dawn approaches. This rise and fall helps coordinate the timing of sleep, but melatonin doesn’t directly cause unconsciousness the way a sedative does. Instead, it opens the “gate” for sleep, making sleep possible and more likely, but not forcing it.
Melatonin influences multiple physiological systems beyond sleep timing. It affects core body temperature regulation, contributing to the slight drop in core temperature that facilitates sleep onset. As melatonin rises, your core temperature drops, while blood flow to your extremities increases (warming your hands and feet through vasodilation). This redistribution of heat from core to periphery is part of the process of falling asleep. If your hands and feet are cold, indicating vasoconstriction, this temperature redistribution can’t occur effectively, which is one reason cold extremities can interfere with sleep initiation.
Melatonin receptors exist throughout the body, not just in the brain but in cardiovascular tissue, immune cells, digestive organs, and more. This widespread distribution suggests melatonin coordinates circadian timing across multiple organ systems. When melatonin rises, it signals to these peripheral clocks that night has arrived, helping keep them synchronised with the master clock in the SCN. This coordination ensures that different body systems are operating on the same schedule.
Melatonin production changes across your lifespan. Young children produce abundant melatonin. Production typically peaks during adolescence, which may seem counterintuitive given teenagers’ reputation for staying up late, but remember that adolescents experience a circadian phase delay where their entire rhythm shifts later, including when melatonin rises. As you age, melatonin production gradually declines. Many elderly individuals produce substantially less melatonin than they did in youth, which partially explains why older adults often experience earlier wake times, lighter sleep, and more difficulty maintaining consolidated sleep through the night.
Now, you may be thinking, “what about melatonin supplements?” Well, they can be useful, but not in the way most people think. Taking melatonin won’t knock you out like a sleeping pill. Instead, it can help shift your circadian timing, which is quite useful for jet lag, adjusting your schedule, or managing certain circadian rhythm disorders. Small doses (0.3-1mg) taken at the right time (typically 2-3 hours before desired bedtime) can help advance your sleep phase if you’re trying to go to bed earlier. Larger doses aren’t necessarily better and may make you feel groggy without improving circadian alignment.
The timing of melatonin supplementation matters enormously. Taking it at the wrong time can actually shift your circadian phase in the wrong direction or have minimal effect. For advancing your schedule (going to bed earlier), take melatonin in the early evening, several hours before your current natural bedtime (this helps shift your DLMO earlier). For delaying your schedule (going to bed later), which is rarely needed, you’d take melatonin in the morning after waking, though this approach is less commonly used and less well-established. But if your sleep problems stem from poor sleep hygiene (excessive light exposure at night, irregular timing, high adenosine from caffeine use), rather than circadian misalignment, melatonin supplements won’t solve them. You’re addressing the wrong system.
Temperature: The Secondary Zeitgeber
As I mentioned earlier, if you were trying to figure out what time of day it was, you may be able to deduce roughly what time it was by virtue of the temperature. Mornings are typically cool, as the sun’s rays haven’t had time to warm the environment. Midday is usually warmest. Evenings can still be quite warm, as the environment retains heat, but temperature gradually drops as night progresses. Your body uses temperature information similarly, though the relationship between temperature and sleep regulation is more complex than it first appears.
The hypothalamus, which houses the SCN, also regulates body temperature. Your core body temperature follows a circadian rhythm (rising during the day and falling at night) largely driven by changes in metabolism and hormonal output as dictated by the SCN. Body temperature typically reaches its lowest point in the early morning hours, begins rising before you wake (helping trigger awakening), continues rising through the day, peaks in the early evening, then declines as bedtime approaches.
Body temperature generally rises in the morning, partly due to cortisol release that wakes you up and increases metabolic activity. As cortisol drops later in the day, body temperature decreases, contributing to sleepiness. This falling core body temperature is actually necessary for sleep initiation, as your body needs to shed heat to fall asleep effectively. Core body temperature typically drops about 0.3-0.5°C from evening peak to overnight trough, though peripheral temperature (in your hands and feet) can change more dramatically as blood flow redistributes from core to extremities.
However, where this gets interesting is that temperature doesn’t primarily influence the master clock in the SCN the way light does. Instead, it affects the peripheral clocks throughout your body. Temperature is a potent zeitgeber for peripheral oscillators in muscle tissue, liver, digestive organs, and other systems. Changes in body temperature can pull these peripheral clocks out of alignment with the master clock. The rest of the body can have its clocks somewhat overridden by temperature signals, potentially ignoring what the SCN says if temperature signals are strong enough or timed inappropriately.
This has significant implications for everyday behaviours:
Room temperature: If your bedroom is too hot or too cold, you’ll struggle to fall asleep and stay asleep. The discomfort is obvious, but there’s also a regulatory component. Temperature extremes send confusing signals to your peripheral clocks. The ideal bedroom temperature for most people is around 16-19°C (60-67°F), cool enough to facilitate the core temperature drop needed for sleep but not so cold as to cause discomfort or vasoconstriction that prevents heat loss from extremities.
Eating and the thermic effect: Eating raises body temperature through the thermic effect of food (this is the energy required to digest, absorb, and process nutrients). This is why eating a large meal too close to bedtime can interfere with sleep. The rise in body temperature from digestion works against the temperature drop your body is trying to achieve for sleep onset. Leaving 2-3 hours between your last substantial meal and sleep gives your body time to process the food and begin its natural temperature decline.
Caloric restriction: Conversely, eating too few calories, particularly in the context of aggressive dieting, can cause cortisol to rise as your body mobilises stored fuel. This cortisol elevation raises body temperature and disrupts sleep. Bodybuilders approaching competition often experience progressively worse sleep as calories drop and body fat becomes very low. Their bodies are in a state of metabolic stress, with elevated cortisol throughout the day and night, raising body temperature and fragmenting sleep.
Stimulants and thermogenesis: Drinking coffee or taking any stimulant raises body temperature through increased metabolic rate and thermogenesis. This temperature elevation contributes to their wakefulness-promoting effects beyond their direct actions on adenosine receptors and other neurotransmitter systems. This is another reason why stimulants consumed too late in the day interfere with sleep; they’re not just blocking adenosine or directly increasing excitation, they’re also raising body temperature when it should be falling.
Exercise timing: Exercise elevates body temperature substantially and triggers cortisol release, especially if the training is intense or prolonged. This is why exercising too close to bedtime can interfere with sleep, particularly for vigorous exercise. Your body temperature remains elevated for several hours after exercise. For most people, finishing exercise at least 2-3 hours before bed allows core temperature to drop appropriately. However, light exercise or gentle movement in the evening typically doesn’t cause problems and may even help some people sleep better.
The warm bath paradox: You might think that a cold shower or bath before bed would help by lowering body temperature directly, but this can actually backfire. Extreme cold can drop your body temperature too rapidly, prompting your body to generate heat to compensate (shivering thermogenesis and metabolic heat production). It can also cause vasoconstriction (narrowing of blood vessels), particularly in your extremities, which impairs your ability to shed heat through your skin.
A warm bath or shower, counterintuitively, may actually be more beneficial. Warm water causes vasodilation (widening of blood vessels), particularly in your hands and feet. This increases blood flow to your extremities, allowing more efficient heat transfer from your core to your periphery and then to the environment. When you get out of the warm bath, your core temperature drops naturally as this vasodilation continues and heat radiates from your now-warm skin. The timing matters here too. Taking a warm bath 60-90 minutes before bed allows this process to unfold optimally, with your core temperature dropping just as you’re preparing for sleep.
Stress and arousal: Stress elevates cortisol and body temperature, contributing to difficulty falling asleep through multiple mechanisms. Chronic stress keeps body temperature elevated throughout the day and prevents the normal circadian temperature decline in the evening. This is one of several pathways by which stress disrupts sleep, but it’s an often-overlooked contributor.
Now, before moving on, it’s important to note that whilst temperature does impact your ability to get to sleep and stay asleep, body temperature is generally quite well regulated through homeostatic mechanisms. We don’t need to excessively concern ourselves with managing every small fluctuation. Your body has sophisticated thermoregulatory systems that usually handle minor variations. However, understanding how your daily activities influence body temperature, and being mindful of the larger patterns like meal timing, exercise timing, stimulant use, and bedroom climate, gives you useful levers for improving sleep. We can certainly make better choices to ensure that our body temperature (and the environment temperature) are at levels conducive to sleep, but we don’t need to become obsessive about achieving perfect temperature control.
Other Zeitgebers: Food, Activity, and Social Cues
Light and temperature are the primary environmental signals that set your circadian clock, but they’re not the only ones. Food timing, physical activity, and social cues also influence your circadian system, though more weakly than light.
Food timing and meal patterns: Regular meal times help entrain circadian rhythms, particularly for peripheral clocks in digestive organs, liver, pancreas, and other metabolic tissues. These organs have their own circadian oscillators that anticipate regular feeding times, pre-emptively increasing enzyme secretion and metabolic activity. Eating at consistent times each day reinforces your body’s internal schedule and keeps peripheral metabolic clocks synchronised with your master clock.
Conversely, erratic meal timing, particularly eating late at night when your body expects to be fasting, can pull peripheral clocks out of sync with the master clock in the SCN. Night-time eating signals to your digestive system that it’s daytime (feeding time), even while your SCN is receiving darkness signals saying it’s night. This desynchronisation between central and peripheral clocks may contribute to metabolic problems associated with shift work and irregular eating patterns.
Time-restricted eating (confining food intake to a consistent window during the day, typically 8-12 hours) may help strengthen circadian rhythms by providing clear, consistent timing signals to metabolic tissues. This is especially likely to be the case if the eating window is shifted to earlier in the day, with a 3-4 hour gap between last meal and sleep. While this is an area of ongoing research, the basic principle makes sense: regular, predictable feeding patterns help keep your internal clocks synchronised.
Exercise timing and activity patterns: Physical activity can also influence circadian rhythms, though the effects are more modest than light. Morning or daytime exercise tends to advance your circadian phase (shifting it earlier), while late evening exercise might delay it (shifting it later). The effect isn’t as powerful as light exposure, but regular exercise at consistent times does help reinforce your daily rhythm.
Exercise may influence circadian timing through multiple mechanisms: temperature changes, hormone release (including cortisol), metabolic signals, and possibly through effects on peripheral clocks in muscle tissue. The practical implication is that maintaining consistent exercise timing (not just exercise frequency and duration) may help stabilise your circadian system.
Social cues and activity schedules: Regular work schedules, social commitments, and alarm clocks act as zeitgebers too, though largely through their influence on your light exposure, activity patterns, and meal timing rather than as direct signals. These social zeitgebers powerfully shape your actual sleep-wake behaviour, even if your biology would prefer different timing. This is the essence of social jet lag, which is when your biological clock says one thing but your social obligations demand another.
Maintaining a consistent daily routine (waking at the same time, eating at regular hours, exercising at predictable times, and engaging in social activities on a schedule) helps keep your circadian system stable and aligned. When all these signals align with each other and with the light-dark cycle, your circadian system runs smoothly. When they conflict (late-night eating, irregular sleep schedules, excessive artificial light at night), your circadian system receives mixed messages and struggles to maintain proper timing.
These secondary zeitgebers work in concert with light and temperature to fine-tune your circadian system. None of them individually can override the powerful signal of light, but collectively they support or undermine your circadian stability depending on whether they’re aligned or in conflict.
Sleep-Wake Homeostasis: The Pressure to Sleep
Circadian rhythms govern the timing of sleep (when you feel sleepy and when you feel alert), but they’re not the only force at play. Sleep-wake homeostasis represents a separate regulatory mechanism that tracks your sleep needs and how much sleep you’ve obtained.
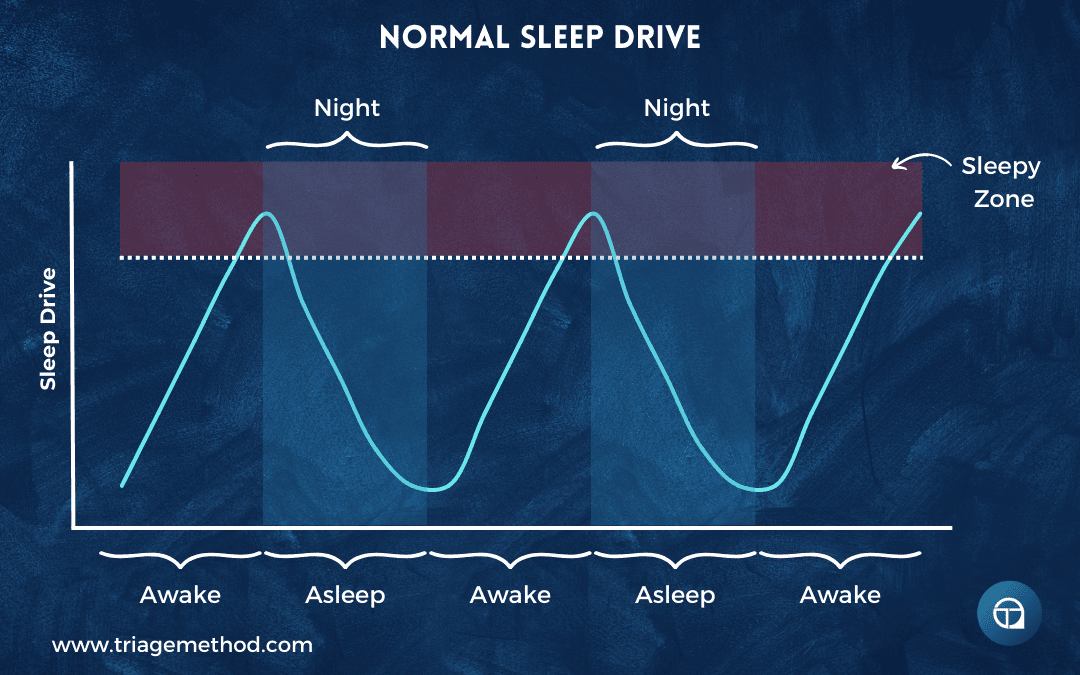
When you’re awake, you build up “sleep drive” or “sleep pressure.” The longer you stay awake, the stronger this pressure grows. When you sleep, the pressure dissipates. This process is independent of circadian rhythms, though the two systems interact extensively.
I find the analogy of a kettle boiling helpful. When you’re awake, you’re heating the kettle. After enough time, it begins bubbling vigorously; this is your signal to remove it from the heat and let it cool (go to sleep). Several common experiences make sense through this lens.
Napping and sleep pressure: Having a nap reduces your sleep drive. If you remove the kettle from the heat mid-day and let it cool (nap), then return it to the heat (resume activity), it takes time to boil again. The water has cooled down, so you need more time heating it before it reaches the boiling point again.
If you nap close to your normal bedtime, you haven’t built up sufficient sleep pressure by evening, making it difficult to fall asleep at your usual time. You might then fail to get adequate sleep because you have a fixed wake time for work, leaving you even more tired the next day. This easily becomes a vicious cycle where you feel you need to nap because you’re exhausted, but the naps themselves prevent adequate nighttime sleep. You’re perpetually working with a partially cooled kettle, never quite reaching the vigorous boil that would allow deep, consolidated nighttime sleep.
Napping earlier in the day gives you more time to rebuild sleep pressure before bed. A nap ending by early afternoon (1-2 pm) leaves 8-10 hours to accumulate sleep drive before a typical 10-11 pm bedtime. Brief naps (20-30 minutes) also minimise the cooling effect; you’re just taking the kettle off the heat briefly rather than letting it cool substantially. Though even early, brief naps don’t work for everyone, and some people find that any napping interferes with night sleep.
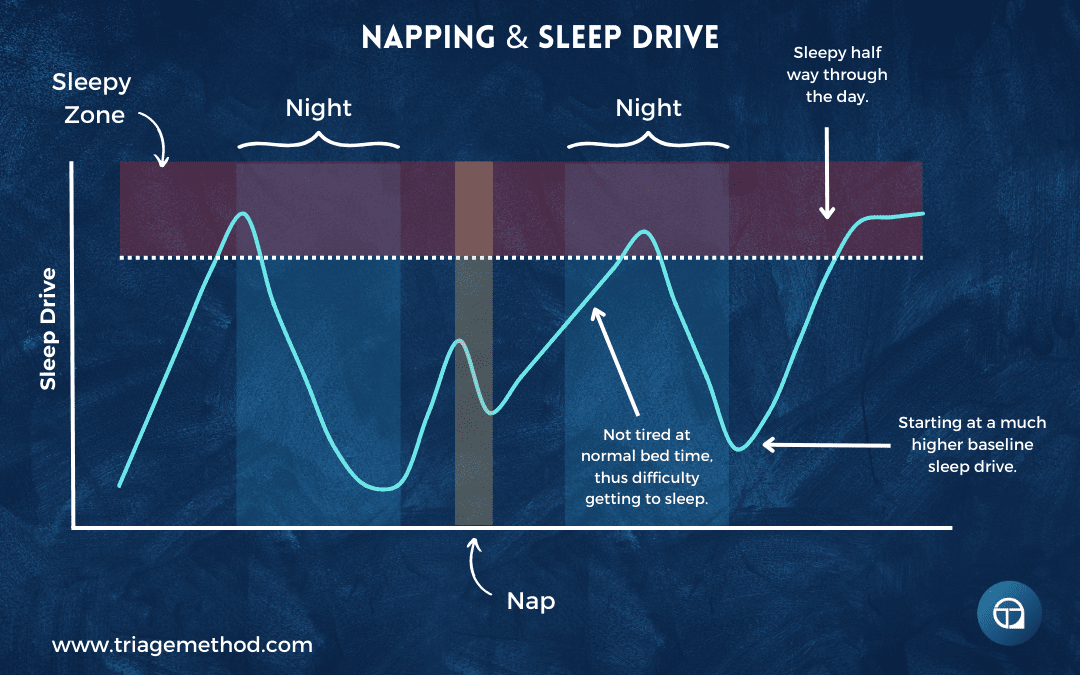
Sleep deprivation and overwhelming pressure: Staying awake all night demonstrates homeostatic sleep pressure overriding circadian signals. You’ve been boiling the kettle for far too long; 16, 20, 24 hours or more of continuous wakefulness. Sleep pressure builds to overwhelming levels, completely uncoupling you from your normal circadian rhythm. You might be trying to stay awake at 3 pm, a time when your circadian system normally promotes alertness, but the massive accumulated sleep debt overrides this circadian wake signal. Your body is screaming for sleep regardless of what time your internal clock says it is.
Chronic sleep restriction and baseline pressure: Chronic sleep restriction creates a persistently warm kettle. If you never give the water sufficient time to cool (never sleep enough), you start each day with elevated baseline sleep pressure. That pressure builds more quickly and intensely throughout the day as sleep debt accumulates across the week.
Imagine you need 8 hours of sleep to fully dissipate sleep pressure, but you only get 6 hours. You begin day two with residual sleep pressure equivalent to 2 hours of wakefulness already accumulated. By the end of day two, you’ve been awake for 16 hours, but your sleep pressure is equivalent to 18 hours of wakefulness. After a week of this pattern, you’re carrying substantial sleep debt, and your baseline sleep pressure is persistently elevated, making you feel tired throughout the day, even shortly after waking.
You might catch up slightly on weekends by sleeping longer, but this pattern disrupts circadian rhythms too, creating social jet lag (again, a mismatch between your biological timing and your social schedule). You’re essentially living in different time zones on weekdays versus weekends, creating further misalignment and perpetuating poor sleep.
Adenosine: The Molecule Behind Sleep Pressure
So, how does sleep-wake homeostasis actually work at a molecular level? The answer centres on adenosine, a molecule you might recognise as part of ATP (adenosine triphosphate), the energy currency of cells. ATP is constantly being broken down during cellular activity, releasing energy and producing adenosine as a byproduct. Adenosine plays a crucial role in sleep regulation, though not in the intuitive way you might expect. It’s not directly about sensing low energy levels.
Throughout waking hours, adenosine accumulates in your brain, particularly in regions involved in arousal and wakefulness like the basal forebrain, cortex, and other areas. Adenosine binds to specific receptors (primarily A1 and A2A receptors) on neurons in these wake-promoting regions. When adenosine binds to A1 receptors, it inhibits wake-promoting neurons, reducing their firing rate. When it binds to A2A receptors in the basal forebrain, it has similar inhibitory effects on arousal systems whilst also promoting sleep-promoting pathways.
The longer you’re awake, the more adenosine accumulates, and the more receptors become occupied, progressively damping down arousal systems and intensifying your sleepiness. It’s a beautifully simple feedback system: wakefulness produces adenosine, adenosine promotes sleep, and sleep clears adenosine.
During sleep, adenosine levels drop and receptors clear. The mechanisms for adenosine clearance aren’t entirely understood, but sleep itself is necessary for this process. Enzymes like adenosine deaminase break down adenosine, and cellular processes remove it from extracellular spaces where it acts on receptors. You wake feeling refreshed after adequate sleep because your adenosine has been cleared and your sleep pressure has been reset.
If you don’t sleep enough, you begin the next day with elevated baseline adenosine and more occupied receptors. You are already starting from a deficit. As the day progresses, adenosine continues accumulating atop this elevated baseline, making you progressively more tired than you would be if you’d slept adequately.
Individual differences exist in adenosine sensitivity and metabolism. Some genetic variants affect adenosine deaminase activity (this is the enzyme that breaks down adenosine). People with more active adenosine deaminase might clear adenosine faster and potentially tolerate sleep restriction better (though still not without consequences). Other genetic variants affect adenosine receptor density or sensitivity, influencing how strongly a given level of adenosine makes you feel sleepy.
The adenosine system also interacts with circadian rhythms. Adenosine accumulation is fairly linear throughout waking hours, but your perception of sleepiness isn’t linear because your circadian system modulates how much adenosine translates into actual sleepiness. During your circadian peak alertness time (typically afternoon for most people), you might have moderate adenosine but not feel particularly sleepy because your circadian arousal system is counteracting it. During your circadian trough (typically early morning hours), the same adenosine level would make you feel very sleepy because your circadian system isn’t providing arousal support.
The only way to truly clear accumulated adenosine is through sleep. You can’t override this system long-term, though you can temporarily mask it, which brings us to caffeine.
Caffeine: Borrowing Wakefulness at a Cost
Caffeine’s molecular structure closely resembles that of adenosine. When you consume caffeine, it travels through your bloodstream, crosses the blood-brain barrier, and competes with adenosine for the same A1 and A2A receptors in your brain. Caffeine binds to these receptors but doesn’t trigger the sleepiness response that adenosine does; it’s like a key that fits the lock but doesn’t turn it. By occupying adenosine receptors, caffeine blocks adenosine from binding, temporarily masking your accumulated sleep pressure.
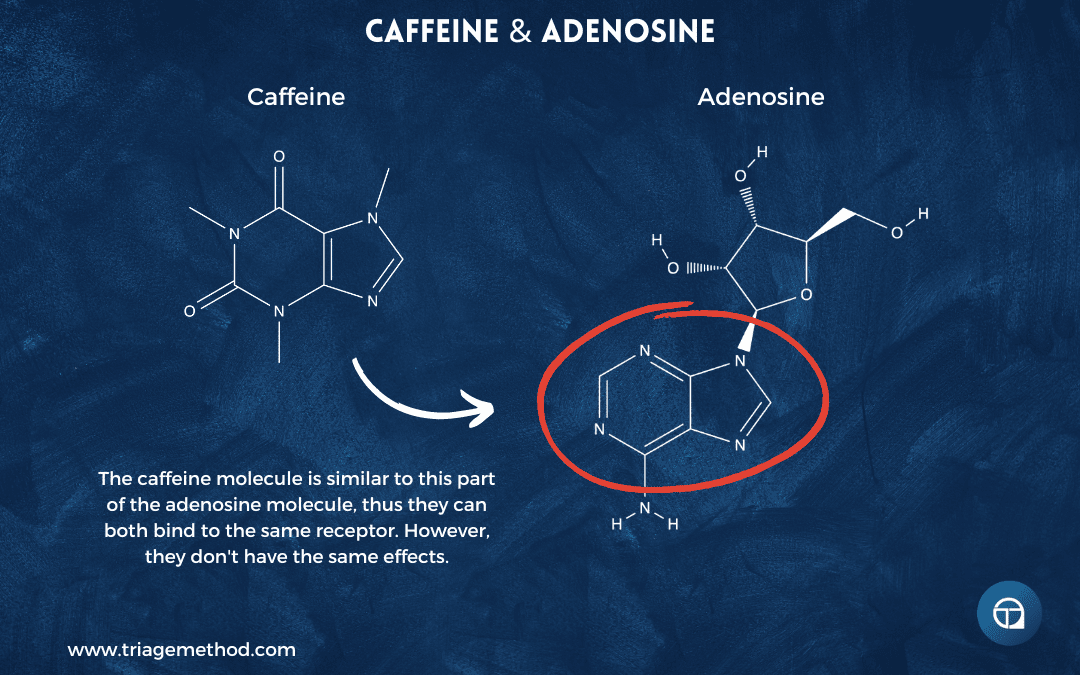
This is why caffeine makes you feel more awake. You haven’t actually reduced your sleep debt or cleared adenosine; you’ve just blocked its effects temporarily. The adenosine is still there, still accumulating. Caffeine has other wakefulness-promoting properties too, including effects on body temperature, increased release of dopamine and other neurotransmitters, and enhanced neural firing patterns, but this adenosine antagonism is central to how it works.
The primary enzyme responsible for breaking down caffeine is CYP1A2, and genetic variants in the gene encoding this enzyme determine whether you’re a fast or slow metaboliser. Fast metabolisers have a caffeine half-life of about 3-4 hours, meaning half the caffeine is eliminated in that time, and most of it (about 94%) is cleared within 12-15 hours. Slow metabolisers might have a half-life of 8-10 hours or longer, meaning caffeine can persist in their system for 24-30 hours before being mostly eliminated. This genetic variation explains why some people can drink coffee in the evening with no apparent effect, while others find caffeine consumed at lunchtime still disrupts their sleep.
Even if you’re a fast metaboliser, caffeine consumed in the afternoon can still affect your sleep architecture that night. Studies show caffeine consumed even 6 hours before bedtime can reduce total sleep time and deep sleep, even in people who report they have “no problem” falling asleep after an afternoon coffee. You might fall asleep, but your sleep quality is compromised. This matters because you’re accumulating sleep debt without realising it. You think you’re sleeping fine, but objective measures show lighter, more fragmented sleep.
Regular caffeine use leads to tolerance too. Your brain upregulates (creates more) adenosine receptors to compensate for chronic blockade, meaning you need more caffeine to achieve the same effect. This is why habitual coffee drinkers often don’t feel “awake” after their morning coffee; they just feel normal. They’re using caffeine to overcome withdrawal symptoms from not having caffeine. Without that morning coffee, they feel tired, foggy, and irritable because their adenosine system has become dependent on caffeine’s presence, not because they’re genuinely short on sleep (though they might be).
Unfortunately, many people chronically undersleep, building up sleep debt and accumulating adenosine. They use caffeine to mask the resulting tiredness, which prevents them from feeling their actual fatigue and making appropriate adjustments (like sleeping more). But adenosine continues accumulating underneath this chemical mask. When the caffeine wears off (typically mid-afternoon), all that accumulated adenosine can hit at once, creating a dramatic energy crash.
The instinctive response is to consume more caffeine, which temporarily solves the immediate problem but pushes the reckoning further into the evening. That second or third coffee makes it harder to fall asleep that night, both through direct adenosine receptor blockade if caffeine is still present at bedtime and through delayed circadian phase from the physiological arousal caffeine creates. You sleep less or worse, accumulate more adenosine the next day, need more caffeine to function, and the cycle perpetuates.
To add insult to injury, caffeine withdrawal can be genuinely unpleasant: headaches, fatigue, irritability, difficulty concentrating, and a whole host of side effects. This is why many people struggle to break the cycle. The withdrawal symptoms typically peak 24-48 hours after your last caffeine, and can last several days to a week. Gradually reducing intake rather than quitting abruptly can minimise withdrawal symptoms.
Hidden sources of caffeine complicate matters. Obviously coffee and tea contain caffeine, but so do energy drinks, many soft drinks (especially cola), chocolate, some pain medications (like Excedrin or Anadin Extra), some cold and flu remedies, pre-workout supplements, and even decaffeinated coffee (which typically contains 2-5mg of caffeine per cup compared to 80-100mg in regular coffee). If you’re trying to reduce caffeine intake or eliminate afternoon caffeine, you need to account for all sources.
Other stimulants interact with the adenosine system too, though through somewhat different mechanisms. Amphetamines and methylphenidate (used for ADHD) enhance dopamine and norepinephrine signalling, promoting wakefulness partly by increasing arousal systems that counteract adenosine’s effects. Modafinil, used for narcolepsy and shift work sleep disorder, appears to work partly through adenosine receptor antagonism along with other mechanisms. Nicotine has complex effects, including some adenosine-related actions. All stimulants, regardless of mechanism, can disrupt sleep if timed inappropriately.
Now, all of this isn’t to say that caffeine is inherently problematic. It isn’t. When used strategically (modest amounts (100-200mg) in the morning, none past early afternoon (2 pm at latest for most people, earlier for slow metabolisers)), it can enhance focus and performance without significantly disrupting sleep. But using caffeine to compensate for chronic sleep debt creates a trap where you’re constantly masking the symptoms of a problem you’re actively making worse.
The Two-Process Model: When Systems Align or Conflict
Understanding sleep regulation means understanding how circadian rhythms (Process C) and sleep-wake homeostasis (Process S) interact. This interaction is called the two-process model of sleep regulation, and it elegantly explains most patterns of sleepiness and alertness you experience.
Imagine your circadian rhythm as a wave that oscillates over 24 hours, creating windows of high sleep propensity (at night, with a smaller dip in early afternoon) and high alertness (morning and later afternoon/early evening peaks). This circadian wake signal is strongest in the early evening, is often called the “wake maintenance zone” or “forbidden zone for sleep,” and typically occurs 2-3 hours before your habitual bedtime.
For someone with an 11 pm habitual bedtime, this wake maintenance zone would fall around 8-9 pm. During this time, even if you’re tired from accumulated sleep pressure, your circadian system powerfully promotes wakefulness, making it difficult to fall asleep. This explains the frustrating experience of feeling exhausted all day, deciding to go to bed early at 9 pm to “catch up,” then lying awake unable to sleep despite your fatigue. You’re trying to sleep during your wake maintenance zone; your homeostatic system is saying “yes, sleep now” through high adenosine levels, but your circadian system is saying “absolutely not, stay awake” through strong arousal signals. The systems are in direct conflict.
Adenosine-driven sleep pressure (Process S) is different, as it builds fairly linearly throughout waking hours. Each hour awake adds more pressure. When you first wake from adequate sleep, sleep pressure is low, and your circadian system is promoting wakefulness (you feel alert). As morning progresses into afternoon, sleep pressure builds, but your circadian alertness signal remains strong enough to counteract it, so you might feel a bit tired but not sleepy. Many people experience an afternoon dip in alertness when rising sleep pressure temporarily overcomes a small circadian trough, though this is often exacerbated by post-lunch blood sugar changes and circadian timing of the dip varies between individuals.
As evening arrives, sleep pressure continues rising whilst your circadian arousal signal begins declining (after passing through the wake maintenance zone). When sleep pressure is high and you’re in the circadian low point for alertness (typically 2-3 hours after your wake maintenance zone ends), sleep comes easily. You fall asleep quickly, descend into deep sleep efficiently, and cycle through stages smoothly. During the night, adenosine clears while your circadian system continues its trough, keeping you asleep. As morning approaches, adenosine has been substantially cleared (reducing sleep pressure) while your circadian system begins ramping up its wake signal, so you naturally wake feeling refreshed.
This elegant coordination breaks down when the systems misalign. Several common scenarios illustrate this:
Late afternoon napping: If you nap at 4-5 pm, you reduce sleep pressure just as your circadian system is preparing for sleep. By the time your normal bedtime arrives, sleep pressure hasn’t rebuilt sufficiently, even though your circadian system is in its sleep zone. You struggle to fall asleep despite being in bed at your usual time, your brain not quite ready for sleep because the homeostatic drive is insufficient.
Night shift work: Working nights means trying to sleep when your circadian system is promoting wakefulness (daytime) and trying to stay awake when it’s promoting sleep (night-time). You’re fighting both systems constantly. Even if you manage to sleep during the day, the quality is often poor because your circadian system is working against you: suppressing melatonin, maintaining higher core body temperature, and promoting arousal. The accumulated adenosine (high sleep pressure from being awake all night) helps you fall asleep during the day, but it’s fighting against your circadian wake signal the entire time.
Jet lag: After flying across time zones, your circadian system remains entrained to your departure location, while your sleep-wake homeostasis responds to your actual wake time in the new location. You might be exhausted (high sleep pressure from being awake many hours), but unable to sleep because your circadian system thinks it’s midday. Or you might feel alert after sleeping because your circadian system thinks it’s morning even though it’s midnight locally. The misalignment between your internal clock and the local time creates the constellation of jet lag symptoms of poor sleep, daytime fatigue, difficulty concentrating, and digestive issues (as peripheral clocks are also misaligned).
Weekend sleep-in and social jet lag: If you normally wake at 6 am on weekdays but sleep until 10 am on weekends, you’re shifting your circadian phase later across the weekend (4 hours is equivalent to flying from London to Dubai). By Monday morning, your circadian system expects to wake at 10 am (local time), but you’re forcing wakefulness at 6 am, effectively giving yourself jet lag every single week. Your sleep pressure may be low enough (because you slept recently), but your circadian system is saying it’s the middle of the night.
“Tired but wired”: This common complaint reflects high sleep pressure (elevated adenosine from insufficient previous sleep) but inappropriate circadian signalling, often from late light exposure, stress-related arousal, or other factors that prevent your circadian system from appropriately transitioning into sleep mode. Your body is saying “I need sleep” through adenosine, but your circadian system is saying “stay alert” through continued arousal signals. The systems are in conflict.
When these systems align properly (consistent sleep-wake timing, appropriate light exposure, adequate sleep duration allowing adenosine clearance), sleep feels effortless, and wakefulness feels natural. When they misalign, you experience the various sleep complaints that plague modern life.
Recovery from circadian misalignment takes time because the circadian system adjusts gradually. For jet lag, the rule of thumb is roughly one day per time zone crossed, meaning your circadian system shifts about one hour per day. You can’t force your SCN to immediately jump to a new schedule; the master clock adjusts through daily accumulated exposure to zeitgebers in the new time zone, gradually shifting its phase until it’s realigned.
You can accelerate this adjustment by strategically using strong zeitgebers, particularly bright light exposure at appropriate times. Light exposure in the early morning hours helps advance your phase (shift earlier), while light exposure in the evening helps delay your phase (shift later). For eastward travel (where you need to advance your clock), seeking bright light exposure in the morning at your destination and avoiding evening light helps. For westward travel (where you need to delay your clock), evening light exposure and avoiding early morning light help. But there are limits to how quickly you can shift, as you’re working with biological systems that evolved to be stable, not rapidly adjustable.
Sleep debt complicates the picture further. When you’ve accumulated significant sleep debt, homeostatic pressure becomes so intense that it can partially override circadian signals. You might fall asleep at inappropriate times (like nodding off during afternoon meetings), because sleep pressure overwhelms your circadian alertness signal. This isn’t your circadian system malfunctioning; it’s your homeostatic system screaming that you desperately need sleep regardless of what time it is.
The two-process model helps explain why “catching up” on sleep on weekends provides some benefit but doesn’t fully compensate for weekday sleep restriction. Sleeping longer on Saturday reduces your accumulated sleep debt (clears adenosine), making you feel better. But it also shifts your circadian phase later, creating misalignment with your Monday morning obligations. The benefit of reduced sleep pressure is partially offset by increased circadian misalignment. This is why maintaining consistent sleep timing, even at the cost of slightly less weekend recovery sleep, often feels better overall. You’re prioritising circadian stability over maximal sleep opportunity.
Circadian Rhythm Disorders: When Your Clock Won’t Sync
For most people, circadian misalignment is temporary. Jet lag, occasional late nights, or periods of poor sleep hygiene that can be corrected. But some individuals experience persistent circadian rhythm disorders where their internal clock either can’t sync to the 24-hour day or is locked into a timing that conflicts with social demands. Understanding these disorders illuminates how fundamental circadian regulation is to normal sleep.
Delayed Sleep Phase Syndrome (DSPS): This represents an extreme version of being a night owl. People with DSPS have circadian systems that are stuck running several hours late relative to societal norms. They can’t fall asleep until 2-6 am, regardless of when they go to bed, and they naturally wake in the late morning or afternoon if allowed to sleep freely. The problem isn’t the amount or quality of their sleep; if they follow their natural timing, they sleep normally. The problem is the profound mismatch between their biology and typical work/school schedules.
DSPS often emerges in adolescence and may persist into adulthood. It’s not simply poor sleep hygiene, though poor sleep habits can worsen it. People with DSPS have genuine difficulty shifting their circadian phase earlier despite good sleep practices. Treatment often involves chronotherapy (gradually shifting sleep times), strategic bright light therapy in the morning, melatonin supplementation in the early evening, and sometimes medications. For some, finding work that accommodates later schedules is more sustainable than constantly battling their biology.
Advanced Sleep Phase Syndrome (ASPS): The opposite of DSPS, ASPS involves a circadian system running too early. People with ASPS become sleepy in the early evening (6-8 pm) and wake in the very early morning (2-4 am). Like DSPS, if they follow their natural timing, they sleep well. The difficulty is social; falling asleep at dinner time and waking before dawn conflicts with normal social and family life. ASPS is more common in older adults and has a genetic component (familial advanced sleep phase has been linked to specific genetic mutations affecting circadian clock genes). Treatment involves evening bright light exposure and sometimes morning melatonin to shift the phase later.
Non-24-Hour Sleep-Wake Disorder: This disorder is most common in completely blind individuals who lack the light input to entrain their circadian clocks to 24 hours. Remember that the natural circadian period is slightly longer than 24 hours (often around 24.2 hours). Without light zeitgebers to reset it daily, the clock free-runs at its natural period. Each day, the person’s sleep timing drifts later, typically by 60-90 minutes for most individuals with this condition, though some variation exists. Over weeks, they cycle through all possible sleep times, sometimes sleeping at night (when they’re temporarily in phase with the external world), sometimes during the day (when completely out of phase). The disorder can occur in sighted individuals too, suggesting their circadian systems are particularly insensitive to light or other zeitgebers. Treatment for blind individuals often involves daily melatonin or the melatonin agonist tasimelteon. For sighted individuals, strict light schedules and melatonin can sometimes help entrain the clock.
Irregular Sleep-Wake Rhythm Disorder: Rather than sleeping in a single consolidated block, people with this disorder sleep in multiple episodes scattered across 24 hours with no clear pattern. This is common in neurodegenerative diseases like Alzheimer’s and Parkinson’s, where disruption of the SCN or its signalling leads to loss of consolidated circadian rhythms. It can also occur with brain injury or developmental disorders. The fragmented sleep is distressing for both the individual and caregivers. Treatment focuses on strengthening zeitgebers (scheduled bright light exposure, timed meals, structured activity) to help reinforce circadian rhythms where possible.
Shift Work Disorder: Working night shifts or rotating shifts forces people to sleep during their circadian day and stay awake during their circadian night. For some shift workers, their circadian systems never fully adapt, and they remain partially entrained to daylight even whilst working nights, creating chronic circadian misalignment. Symptoms include difficulty sleeping during the day despite adequate opportunity, excessive sleepiness during night shifts, and various health consequences (shift work is associated with increased risks of cardiovascular disease, metabolic disorders, and some cancers, likely mediated through chronic circadian disruption). Some people adapt better than others based on individual differences in circadian sensitivity. Treatment involves optimising shift schedules where possible (permanent night shifts adapt better than rotating shifts), strategic light exposure (bright light during night shifts, darkness when trying to sleep during the day), and sometimes melatonin or other medications.
Jet Lag Disorder: Whilst jet lag is temporary for most people, frequent travellers crossing many time zones can experience persistent symptoms. The circadian system takes time to adjust (roughly one day per time zone crossed), so if you travel before fully adjusting, you accumulate circadian debt. Eastward travel (requiring phase advance) is typically harder than westward travel (phase delay) because it’s easier to stay up later than force earlier sleep. Symptoms include sleep disturbance, daytime fatigue, impaired cognition, digestive issues, and mood changes. Management involves strategic light exposure, melatonin timing, and sometimes planned sleep schedules before travel to pre-adapt partially.
These disorders illustrate that the circadian system isn’t infinitely flexible. You can’t simply decide to sleep at arbitrary times through willpower. The SCN follows its own logic based on evolutionary programming and the zeitgebers it receives. When that internal logic conflicts with external demands (whether from extreme chronotype, loss of light input, neurological damage, or work schedules), the result is genuine suffering and health consequences.
For people with circadian rhythm disorders, the advice to “just go to bed earlier” or “just wake up earlier” is not only unhelpful but reveals a fundamental misunderstanding of how sleep regulation works. These aren’t behavioural choices; they’re biological limitations. Treatment requires either shifting the circadian system through targeted interventions (light, melatonin, scheduled behaviours) or, where that fails, accommodating the individual’s biological timing rather than forcing conformity to social norms.
Chronotypes: Real Variation or Poor Sleep Hygiene?
As we’ve discussed circadian rhythms and disorders, the question of chronotypes inevitably arises. Your chronotype describes where you fall on the spectrum from extreme morning lark to extreme night owl, essentially, how your individual circadian system is timed relative to the average person. This is genuine biological variation with deep evolutionary logic: in a communal group, having different members naturally alert at different times provides survival advantages by ensuring someone is always watchful.
Chronotype exists on a continuum. At one extreme are morning larks who naturally wake early, feel most alert and capable in the morning, and become sleepy early in the evening. At the other extreme are night owls who struggle with early mornings, feel foggy until late morning or afternoon, hit their stride in the evening, and naturally prefer late bedtimes. Most people cluster toward the middle of the distribution, neither strongly morning nor evening oriented, often called intermediate types or “hummingbirds.”
There are genetic variants associated with chronotype, including differences in the PER3 gene and other clock genes like CLOCK and BMAL1. Some variants lead to shorter circadian periods (predisposing toward morning types), while others lead to longer periods (predisposing toward evening types). These genetic differences affect the fundamental timing of your circadian oscillator in the SCN.
However, while “night owl” has become an extremely popular self-identification, it is often claimed by people who are simply practising poor sleep hygiene rather than expressing an authentic late chronotype. If you stay up late scrolling your phone, exposing yourself to bright blue light, drinking coffee in the evening, eating late meals, then naturally struggle to wake early the next morning, you’re not necessarily a night owl. You’re experiencing the predictable consequences of behaviours that delay your circadian phase and prevent adequate sleep.
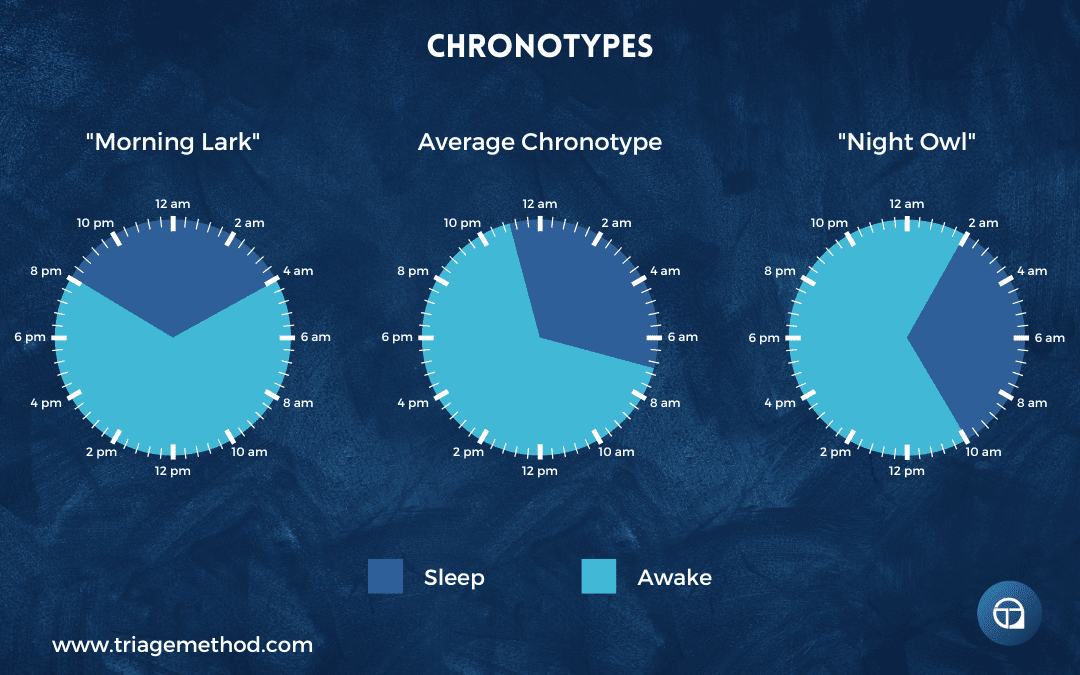
True night owls have circadian systems that naturally run later even with exemplary sleep practices (consistent sleep-wake times, appropriate light exposure, no evening stimulants, etc.). They genuinely feel most alert and capable later in the day, reaching peak cognitive performance in the evening when most people are winding down. Their DLMO (dim light melatonin onset) occurs later than average, even when they’re not engaging in circadian-disrupting behaviours.
Before self-diagnosing as a night owl, you need to honestly assess your sleep hygiene. Implement proper sleep practices consistently for 4-6 weeks: consistent sleep-wake times (including weekends), morning bright light exposure, dimming lights in the evening, no screens for 1-2 hours before bed, no caffeine after noon, regular meal times, and bedroom optimised for sleep. After this period of optimal circadian hygiene, observe your natural tendencies. Do you still struggle with mornings despite these practices? Do you still feel notably better with a later schedule? If so, you’re probably authentically on the later end of the chronotype spectrum.
Many people who think they’re night owls find their “natural” chronotype shifts substantially earlier when they implement proper sleep practices consistently. Their perceived night owl tendency was actually circadian phase delay caused by behaviour, not intrinsic biology. This doesn’t mean extreme night owls don’t exist; they absolutely do, but they’re rarer than self-identification would suggest.
Chronotype also changes across your lifespan, following a fairly predictable pattern. Young children tend toward earlier timing, and anyone who has a toddler will be well aware of the stereotypical early-rising toddler phenomenon. As children enter adolescence, they experience a dramatic shift toward later timing. This isn’t laziness, rebellion, or poor choices; it’s a genuine biological shift in circadian phase that occurs during puberty. Teenagers’ circadian systems naturally shift 2-3 hours later on average, meaning a teenager’s midnight bedtime and 9 am wake time is biologically equivalent to a 9-10 pm bedtime and 6-7 am wake time for adults.
This adolescent phase delay creates profound conflict with early school start times. Forcing teenagers to wake at 6 am for 7-8 am school starts is requiring them to function during what their biology considers the middle of the night. This contributes to widespread sleep deprivation in teenagers, with consequences for learning, mood, mental health, risk-taking behaviour, and physical health. Schools that have shifted to later start times (9:00 am or even later) consistently report improved attendance, grades, and reduced car accidents among teen drivers.
As people move through their twenties, their chronotype typically becomes somewhat earlier. Middle-aged adults often find they’re naturally waking earlier than they did as young adults. Elderly individuals frequently shift substantially earlier (the stereotypical early-rising grandparent). This advanced phase in older adults partly reflects changes in circadian system strength (weaker circadian amplitude, earlier phase), reduced melatonin production, and other age-related changes. However, maintaining good sleep practices, regular exercise, and cognitive engagement can help preserve more flexible timing even in older age.
Unfortunately, modern society is heavily biased toward morning schedules. Work and school typically begin at 8-9 am and end at 5-6 pm, which works well for morning larks and intermediate types but disadvantages genuine evening types. People on the later end of the chronotype spectrum find themselves in constant conflict with social demands, forced to wake earlier than their biology prefers. This chronic circadian misalignment has been called “social jet lag”. You are effectively living in one biological time zone while society operates on another.
This creates pressure for evening types to constantly rely on alarm clocks, caffeine, and willpower to function during their biological night, while fighting sleepiness during their evening alertness period to accommodate social norms. The accumulated sleep debt and circadian misalignment contribute to various health problems that are more common in evening types. Being an evening type isn’t intrinsically unhealthy, but because society forces them into chronic misalignment, they experience ill effects.
This is one reason many self-identified night owls gravitate toward flexible industries like technology, creative fields, or shift work that accommodates later schedules. Finding work arrangements that match your chronotype (or at least don’t force extreme misalignment) serves your health better than constantly battling your biology.
Now, you may be wondering whether you can change your chronotype. And the answer is, somewhat, but not completely. The genetic component sets boundaries on how far you can shift. A true extreme evening type cannot become a morning lark through behaviour change alone, though they might shift from “very late” to “moderately late.” Similarly, an extreme morning lark won’t become a night owl. But most people have some flexibility within a range of 1-2 hours through consistent circadian practices.
The key is working with your chronotype rather than fighting it where possible, while recognising that strong sleep hygiene can shift you within your natural range. If you’re intermediate, you likely have substantial flexibility to adjust timing as needed. If you’re on one extreme, accept that reality and structure your life accordingly rather than engaging in perpetual warfare with your circadian system.
Age, Sex, and Individual Variation in Sleep Regulation
Sleep regulation isn’t identical across all people. Age produces some of the most dramatic differences. Infants have very different sleep-wake patterns than adults. They typically have shorter sleep cycles (50-60 minutes versus 90-120 minutes), more REM sleep (sometimes 50% of sleep versus 20-25% in adults), and more frequent sleep periods distributed across 24 hours. Their circadian systems are still developing, gradually consolidating sleep into longer nighttime periods over the first months and years of life.
As previously discussed, adolescents experience a genuine circadian phase delay during puberty, and their natural tendency shifts 1-3 hours later regardless of behaviour. This reflects changes in circadian period, sensitivity to evening light, and other neurobiological changes occurring during this developmental period. Forcing teenagers to wake at 6 am for school is fighting their biology, contributing to widespread sleep deprivation with consequences for learning, mood, mental health, and physical health.
Adults typically have the most stable circadian patterns, with peak stability generally occurring in the 30s-50s. However, individual variation in chronotype and sleep need persists. Some adults are morning types, others evening types, and most are in between. Sleep need also varies, with most adults requiring 7-9 hours but some functioning well on slightly more or slightly less.
Elderly individuals often experience phase advancement (shifting earlier), increased sleep fragmentation (more awakenings through the night), reduced deep sleep, earlier wake times, and sometimes difficulty falling asleep. These changes partly reflect weakening of the circadian system (reduced amplitude of circadian rhythms), reduced melatonin production, changes in adenosine sensitivity, and various other age-related neurobiological changes. However, maintaining cognitive engagement, physical activity, social connections, and good sleep practices helps preserve sleep quality in older adults. Decline isn’t inevitable, though some degree of change is common.
Sex differences exist too, particularly relating to hormonal fluctuations. The menstrual cycle affects sleep in many women, with some experiencing worse sleep during the luteal phase (after ovulation) when progesterone rises, then falls, or during menstruation when both estrogen and progesterone are low. The relationship is complex and varies between individuals, but hormonal fluctuations influence body temperature regulation, melatonin secretion, and sleep architecture.
Pregnancy dramatically alters sleep, particularly in later stages. Physical discomfort, frequent urination, hormonal changes, fetal movements, and anxiety about impending parenthood all contribute. The first trimester often brings increased sleepiness due to rising progesterone. The third trimester typically brings sleep fragmentation and reduced deep sleep due to discomfort and fetal activity. Postpartum sleep is profoundly disrupted by infant care needs, hormonal shifts, and, often, mood changes.
Menopause often disrupts sleep through multiple mechanisms, including hot flushes (vasomotor symptoms that wake you from sleep), hormonal changes (declining estrogen and progesterone), mood changes (anxiety and depression), and physical changes (increased risk of sleep apnoea). Hormone replacement therapy can sometimes improve sleep by managing hot flushes, though the decision involves weighing various health considerations.
Women appear somewhat more vulnerable to insomnia than men, with roughly 1.5-2 times higher prevalence. This may relate to hormonal factors, differences in stress responses, higher rates of anxiety and depression in women, or other factors. However, men are more likely to develop obstructive sleep apnoea, partly due to anatomical differences in upper airway structure and fat distribution patterns.
Individual genetic variation affects how much sleep you need, how sensitive you are to sleep deprivation, and how quickly you recover from sleep debt. Some rare genetic variants (including mutations in DEC2, ABCC9, and BHLHE41 genes) allow people to function well on 5-6 hours of sleep without apparent consequences. These are genuine “short sleepers,” whose bodies apparently accomplish in 5-6 hours what most people require 7-9 hours to achieve. This is extremely rare, affecting perhaps 1% of the population.
For the vast majority of people, claims of thriving on minimal sleep are either self-deception (they’re tolerating impairment without recognising it), denial of actual consequences (they feel fine but objective performance and health measures reveal deficits), or involve unsustainable reliance on stimulants (masking sleep debt with caffeine or other substances). The myth that you can train yourself to need less sleep is particularly pernicious and has likely contributed to the glorification of sleep deprivation in certain work cultures.
While you might adapt somewhat to chronic sleep restriction (e.g. feeling less acutely terrible than initially), objective performance measures reveal ongoing deficits in reaction time, decision-making, emotional regulation, creativity, and learning. Health markers show increased inflammation, impaired glucose metabolism, elevated cardiovascular risk, and weakened immune function. You’re tolerating impairment, not eliminating it. The body doesn’t become more efficient at sleep under restriction; it simply operates with insufficient resources while deteriorating slowly.
Environmental and Behavioural Disruption in Modern Life
Understanding sleep regulation clarifies why modern life is so hostile to sleep. Our circadian systems evolved to respond to natural light cycles: bright days with light intensity reaching 100,000 lux, dark nights with essentially 0 lux aside from starlight and moon, with gradual transitions at dawn and dusk as the sun’s angle and atmospheric filtering change. We now have artificial light at all hours, with indoor environments typically 100-500 lux (barely adequate to maintain circadian entrainment) during the day and often 50-200 lux in the evening when our brains expect darkness below 10 lux. We have a particular abundance of blue-wavelength light from LED bulbs and screens precisely when our brains expect red-shifted, dim light or darkness.
This inverted light exposure pattern of inadequate brightness during the day and excessive brightness at night creates weak, confused circadian signals. The dim indoor light during the day provides insufficient signal to strongly entrain your SCN, while evening light suppresses melatonin and delays circadian phase. The result is a circadian system that’s weakly anchored and easily disrupted.
Irregular schedules (varying bedtimes, late-night activities, weekend sleep-ins, shift work, etc.) constantly disrupt circadian entrainment. Our sleep-wake homeostasis evolved assuming consistent daily patterns, not caffeine-fuelled sprints through the week followed by crash recovery weekends. The chronic mismatch between biological timing and social obligations (social jet lag) creates perpetual circadian misalignment.
Screen time in the evening is particularly problematic because devices emit substantial blue light at close range directly into your eyes, precisely the wavelength and positioning most effective at suppressing melatonin and delaying circadian phase. The content on screens is often stimulating (social media, news, games, work), creating psychological arousal that compounds the light exposure problem. Checking your phone at 11 pm sends powerful “it’s daytime” signals to your SCN whilst simultaneously activating arousal systems with whatever you’re viewing.
Many medications affect sleep regulation, though doctors don’t always mention sleep effects when prescribing. Stimulant medications for ADHD obviously promote wakefulness. Many antidepressants affect sleep architecture, with SSRIs often suppressing REM sleep and some causing insomnia, while others cause sedation. Beta-blockers can suppress melatonin production. Corticosteroids promote wakefulness and can severely disrupt sleep. Antihistamines cause drowsiness (they’re the active ingredient in many over-the-counter sleep aids), though they don’t produce normal sleep architecture and can cause next-day grogginess. If you’re on medication and experiencing sleep problems, discussing this with your doctor is worthwhile (and you should never discontinue prescribed medications without medical guidance).
Substance use profoundly disrupts sleep regulation. We’ve already discussed caffeine extensively. Alcohol deserves particular mention: while it might help you fall asleep initially by increasing GABA activity (sedation), it severely disrupts sleep architecture. Alcohol suppresses REM sleep in the first half of the night, and fragments sleep in the second half as it metabolises. You often experience a REM rebound in early morning as alcohol clears, leading to vivid, disturbing dreams. The result is sleep that looks adequate in duration but is poor in quality, and you wake feeling unrefreshed despite “eight hours” in bed.
Cannabis also affects sleep in complex ways that aren’t fully understood, but it likely suppresses REM sleep with chronic use, similarly to alcohol. Many people report that cannabis helps them fall asleep, which may be accurate acutely, but chronic use can create dependence and reduce sleep quality. Other recreational drugs have their own effects, almost universally detrimental to sleep quality when examined objectively.
Stress and anxiety interfere with sleep through multiple pathways. Chronic stress elevates cortisol, which raises body temperature and promotes arousal, working against the circadian decline in arousal that should occur in the evening. Psychological stress increases cognitive and emotional arousal (racing thoughts, worry, rumination, etc.), preventing the mental quieting necessary for sleep onset. The hyperarousal characteristic of chronic stress can prevent descent into deep sleep stages and cause frequent awakenings. Chronic stress can create a self-reinforcing cycle where poor sleep increases stress reactivity, which further degrades sleep.
Pain disrupts sleep both by causing frequent awakenings and by preventing deep sleep stages. Chronic pain sufferers often show reduced deep sleep and increased light sleep, presumably because pain signals prevent the nervous system from fully downregulating into deep sleep states. Addressing underlying pain conditions (whether through medical treatment, physical therapy, psychological approaches like CBT, or other means) often improves sleep as a secondary benefit.
Working With Your Biology, Not Against It
Understanding sleep regulation reveals the uncomfortable fact that you cannot hack, override, or trick these systems long-term. Your circadian clock responds to environmental signals following evolutionary logic millions of years old. Adenosine accumulates during waking and clears during sleep according to biological necessity. You can work with these systems or fight against them, but you cannot eliminate them.
This might sound limiting, but it’s actually liberating. Rather than searching for shortcuts or trying to become one of those mythical people who thrive on four hours of sleep, you can focus on aligning your behaviour with how these systems actually work. You have genuine control here. This is classic dichotomy of control. You don’t have control over the systems themselves, but you do have control over the inputs they respond to.
The practical implications flow naturally from understanding these mechanisms:
Light exposure patterns matter enormously. Getting bright light early in the day (ideally natural sunlight, but bright indoor light works too) helps set your circadian clock appropriately and advances your phase (shifts it earlier). Morning light exposure of 30-60 minutes, ideally within an hour or two of waking, provides strong zeitgeber signals. Dimming lights in the evening as bedtime approaches allows melatonin to rise naturally. Minimising blue light exposure in the 1-3 hours before bed prevents melatonin suppression. This isn’t about perfectionism, and it doesn’t mean going out and getting expensive blue-blocking glasses; it’s just about working in the direction your biology already wants to go.
Consistency in timing helps both circadian and homeostatic systems. Regular sleep-wake times, even on weekends (within an hour or so), keep your circadian clock stable rather than forcing it to constantly adjust. Consistent timing also ensures you’re building and clearing sleep pressure on a predictable schedule rather than creating chronic misalignment. Your body performs better with consistency than with perfect optimisation on any given night.
Managing adenosine accumulation means recognising that sleep is non-negotiable. This means using caffeine strategically, rather than as a substitute for adequate sleep. It also means understanding that naps reduce nighttime sleep pressure and timing them accordingly (early and brief if you must nap). And it definitely means respecting that sleep debt accumulates and must be repaid, that you can’t simply push through indefinitely on willpower and stimulants.
Temperature considerations are usually straightforward in modern environments with climate control, but awareness of how meals, exercise, and substances affect body temperature helps with timing these activities appropriately. Finishing vigorous exercise and large meals 2-3 hours before bed allows body temperature to drop appropriately. Keeping your bedroom cool (16-19°C) facilitates heat dissipation. Understanding that a warm bath 60-90 minutes before bed can facilitate a subsequent temperature drop.
You cannot completely override your chronotype, but you can work with it. If you’re genuinely on the later end of the spectrum, seeking work and lifestyle arrangements that accommodate this serves you better than forcing early schedules and relying on stimulants to function. If you’re an intermediate type, you likely have flexibility to adjust timing as needed. If you’re a morning type, don’t force yourself to stay up late trying to match social norms.
The goal isn’t perfection. Your sleep regulation systems are robust and can tolerate occasional disruptions like late nights for social events, travel, periods of high stress, and occasional shift work when necessary. What they struggle with is chronic misalignment between your behaviour and their natural rhythms. Small consistent adjustments in the right direction produce better results than sporadic perfect adherence followed by complete abandonment. Avoid the all-or-nothing mentality here.
Sleep Regulation Conclusion: Two Systems, One Goal
Sleep regulation might seem complex as there are two interacting systems, multiple brain regions, various neurotransmitters and hormones, environmental signals, internal clocks, master clocks and peripheral clocks, genetic variants and individual differences. But the fundamental logic is straightforward. Your circadian system attempts to sync your biology to the external day-night cycle, creating appropriate timing for sleep and wakefulness. Your homeostatic system tracks sleep debt and recovery, creating pressure to sleep that builds during waking and dissipates during sleep.
When these systems align (i.e. when your behaviour supports rather than fights your biology) sleep becomes straightforward. You feel naturally tired at bedtime, fall asleep easily, sleep soundly through the night, and wake refreshed and alert. When they misalign (usually through poor light management, irregular timing, inappropriate caffeine use, chronic sleep restriction, or sometimes even circadian rhythm disorders) sleep becomes a struggle despite being a fundamental biological necessity.
Understanding the “why” behind sleep regulation isn’t just intellectually satisfying. It is actually useful in transforming your relationship with sleep from something that either works or doesn’t into a system you can influence through daily choices. The understanding of sleep regulation allows you to understand how to create the conditions that allow your natural regulatory systems to function as they evolved to.
In the next article, we’ll address the practical question that follows naturally from understanding sleep regulation: how much sleep do you actually need? Understanding the systems that control sleep sets the stage for making informed decisions about sleep duration, timing, and quality.
As with everything, there is always more to learn, and we haven’t even begun to scratch the surface with all this stuff. However, if you are interested in staying up to date with all our content, we recommend subscribing to our newsletter and bookmarking our free content page. We do have a lot of content on sleep in our sleep hub.
The previous article in this series was Understanding Sleep Architecture: What Actually Happens When You Sleep, and the next article in this series is How Much Sleep Do You Need?
If you would like more help with your training (or nutrition), we do also have online coaching spaces available.
We also recommend reading our foundational nutrition articles, along with our foundational articles on exercise and stress management, if you really want to learn more about how to optimise your lifestyle. If you want even more free information on sleep, you can follow us on Instagram, YouTube or listen to the podcast, where we discuss all the little intricacies of exercise.
Finally, if you want to learn how to coach nutrition, then consider our Nutrition Coach Certification course. We do also have an exercise program design course, if you are a coach who wants to learn more about effective program design and how to coach it. We do have other courses available too, notably a sleep course. If you don’t understand something, or you just need clarification, you can always reach out to us on Instagram or via email.
References and Further Reading
Vyazovskiy, V. (2015). Sleep, recovery, and metaregulation: explaining the benefits of sleep. Nature and Science of Sleep, 171. http://doi.org/10.2147/nss.s54036
Sharma, S., & Kavuru, M. (2010). Sleep and Metabolism: An Overview. International Journal of Endocrinology, 2010, 1–12. http://doi.org/10.1155/2010/270832
Yoo, S.-S., Gujar, N., Hu, P., Jolesz, F. A., & Walker, M. P. (2007). The human emotional brain without sleep — a prefrontal amygdala disconnect. Current Biology, 17(20). http://doi.org/10.1016/j.cub.2007.08.007
Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol. 2005;6(6):341-7. PMID: 16459757. https://pubmed.ncbi.nlm.nih.gov/16459757/
Spiegel, K., Leproult, R., L’Hermite-Balériaux, M., Copinschi, G., Penev, P. D., & Cauter, E. V. (2004). Leptin Levels Are Dependent on Sleep Duration: Relationships with Sympathovagal Balance, Carbohydrate Regulation, Cortisol, and Thyrotropin. The Journal of Clinical Endocrinology & Metabolism, 89(11), 5762–5771. http://doi.org/10.1210/jc.2004-1003
Nedeltcheva, A. V., Kilkus, J. M., Imperial, J., Kasza, K., Schoeller, D. A., & Penev, P. D. (2008). Sleep curtailment is accompanied by increased intake of calories from snacks. The American Journal of Clinical Nutrition, 89(1), 126–133. http://doi.org/10.3945/ajcn.2008.26574
Mullington, J. M., Chan, J. L., Dongen, H. P. A. V., Szuba, M. P., Samaras, J., Price, N. J., … Mantzoros, C. S. (2003). Sleep Loss Reduces Diurnal Rhythm Amplitude of Leptin in Healthy Men. Journal of Neuroendocrinology, 15(9), 851–854. http://doi.org/10.1046/j.1365-2826.2003.01069.x
Leproult, R., & Cauter, E. V. (2009). Role of Sleep and Sleep Loss in Hormonal Release and Metabolism. Pediatric Neuroendocrinology Endocrine Development, 11–21. http://doi.org/10.1159/000262524
Spaeth, A. M., Dinges, D. F., & Goel, N. (2013). Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep, 36(7), 981–990. http://doi.org/10.5665/sleep.2792
Calvin, A. D., Carter, R. E., Adachi, T., Macedo, P. G., Albuquerque, F. N., Walt, C. V. D., … Somers, V. K. (2013). Effects of Experimental Sleep Restriction on Caloric Intake and Activity Energy Expenditure. Chest, 144(1), 79–86. http://doi.org/10.1378/chest.12-2829
Markwald, R. R., Melanson, E. L., Smith, M. R., Higgins, J., Perreault, L., Eckel, R. H., & Wright, K. P. (2013). Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences, 110(14), 5695–5700. http://doi.org/10.1073/pnas.1216951110
Cauter, E. V., Spiegel, K., Tasali, E., & Leproult, R. (2008). Metabolic consequences of sleep and sleep loss. Sleep Medicine, 9. http://doi.org/10.1016/s1389-9457(08)70013-3
Spiegel, K., Leproult, R., & Cauter, E. V. (1999). Impact of sleep debt on metabolic and endocrine function. The Lancet, 354(9188), 1435–1439. http://doi.org/10.1016/s0140-6736(99)01376-8
Ness, K. M., Strayer, S. M., Nahmod, N. G., Schade, M. M., Chang, A.-M., Shearer, G. C., & Buxton, O. M. (2019). Four nights of sleep restriction suppress the postprandial lipemic response and decrease satiety. Journal of Lipid Research, 60(11), 1935–1945. http://doi.org/10.1194/jlr.p094375
Hirotsu, C., Tufik, S., & Andersen, M. L. (2015). Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Science, 8(3), 143–152. http://doi.org/10.1016/j.slsci.2015.09.002
Morselli, L., Leproult, R., Balbo, M., & Spiegel, K. (2010). Role of sleep duration in the regulation of glucose metabolism and appetite. Best Practice & Research Clinical Endocrinology & Metabolism, 24(5), 687–702. http://doi.org/10.1016/j.beem.2010.07.005
Lamon, S., Morabito, A., Arentson-Lantz, E., Knowles, O., Vincent, G. E., Condo, D., … Aisbett, B. (2020). The effect of acute sleep deprivation on skeletal muscle protein synthesis and the hormonal environment. http://doi.org/10.1101/2020.03.09.984666
Lipton, J. O., & Sahin, M. (2014). The Neurology of mTOR. Neuron, 84(2), 275–291. http://doi.org/10.1016/j.neuron.2014.09.034
Tudor, J. C., Davis, E. J., Peixoto, L., Wimmer, M. E., Tilborg, E. V., Park, A. J., … Abel, T. (2016). Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Science Signaling, 9(425). http://doi.org/10.1126/scisignal.aad4949
Dattilo, M., Antunes, H., Medeiros, A., Neto, M. M., Souza, H., Tufik, S., & Mello, M. D. (2011). Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Medical Hypotheses, 77(2), 220–222. http://doi.org/10.1016/j.mehy.2011.04.017
Thornton, S. N., & Trabalon, M. (2014). Chronic dehydration is associated with obstructive sleep apnoea syndrome. Clinical Science, 128(3), 225–225. http://doi.org/10.1042/cs20140496
Rosinger, A. Y., Chang, A.-M., Buxton, O. M., Li, J., Wu, S., & Gao, X. (2018). Short sleep duration is associated with inadequate hydration: cross-cultural evidence from US and Chinese adults. Sleep, 42(2). http://doi.org/10.1093/sleep/zsy210
Watson, A. M. (2017). Sleep and Athletic Performance. Current Sports Medicine Reports, 16(6), 413–418. http://doi.org/10.1249/jsr.0000000000000418
Bonnar, D., Bartel, K., Kakoschke, N., & Lang, C. (2018). Sleep Interventions Designed to Improve Athletic Performance and Recovery: A Systematic Review of Current Approaches. Sports Medicine, 48(3), 683–703. http://doi.org/10.1007/s40279-017-0832-x
Saidi, O., Davenne, D., Lehorgne, C., & Duché, P. (2020). Effects of timing of moderate exercise in the evening on sleep and subsequent dietary intake in lean, young, healthy adults: randomized crossover study. European Journal of Applied Physiology, 120(7), 1551–1562. http://doi.org/10.1007/s00421-020-04386-6
Abedelmalek, S., Chtourou, H., Aloui, A., Aouichaoui, C., Souissi, N., & Tabka, Z. (2012). Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. European Journal of Applied Physiology, 113(1), 241–248. http://doi.org/10.1007/s00421-012-2432-7
Azboy, O., & Kaygisiz, Z. (2009). Effects of sleep deprivation on cardiorespiratory functions of the runners and volleyball players during rest and exercise. Acta Physiologica Hungarica, 96(1), 29–36. http://doi.org/10.1556/aphysiol.96.2009.1.3
Bird, S. P. (2013). Sleep, Recovery, and Athletic Performance. Strength and Conditioning Journal, 35(5), 43–47. http://doi.org/10.1519/ssc.0b013e3182a62e2f
Blumert, P. A., Crum, A. J., Ernsting, M., Volek, J. S., Hollander, D. B., Haff, E. E., & Haff, G. G. (2007). The Acute Effects of Twenty-Four Hours of Sleep Loss on the Performance of National-Caliber Male Collegiate Weightlifters. The Journal of Strength and Conditioning Research, 21(4), 1146. http://doi.org/10.1519/r-21606.1
Chase, J. D., Roberson, P. A., Saunders, M. J., Hargens, T. A., Womack, C. J., & Luden, N. D. (2017). One night of sleep restriction following heavy exercise impairs 3-km cycling time-trial performance in the morning. Applied Physiology, Nutrition, and Metabolism, 42(9), 909–915. http://doi.org/10.1139/apnm-2016-0698
Edwards, B. J., & Waterhouse, J. (2009). Effects of One Night of Partial Sleep Deprivation upon Diurnal Rhythms of Accuracy and Consistency in Throwing Darts. Chronobiology International, 26(4), 756–768. http://doi.org/10.1080/07420520902929037
Fullagar, H. H. K., Skorski, S., Duffield, R., Hammes, D., Coutts, A. J., & Meyer, T. (2014). Sleep and Athletic Performance: The Effects of Sleep Loss on Exercise Performance, and Physiological and Cognitive Responses to Exercise. Sports Medicine, 45(2), 161–186. http://doi.org/10.1007/s40279-014-0260-0
Gupta, L., Morgan, K., & Gilchrist, S. (2016). Does Elite Sport Degrade Sleep Quality? A Systematic Review. Sports Medicine, 47(7), 1317–1333. http://doi.org/10.1007/s40279-016-0650-6
Hausswirth, C., Louis, J., Aubry, A., Bonnet, G., Duffield, R., & Meur, Y. L. (2014). Evidence of Disturbed Sleep and Increased Illness in Overreached Endurance Athletes. Medicine & Science in Sports & Exercise, 46(5), 1036–1045. http://doi.org/10.1249/mss.0000000000000177
Mah, C. D., Mah, K. E., Kezirian, E. J., & Dement, W. C. (2011). The Effects of Sleep Extension on the Athletic Performance of Collegiate Basketball Players. Sleep, 34(7), 943–950. http://doi.org/10.5665/sleep.1132
Milewski, M. D., Skaggs, D. L., Bishop, G. A., Pace, J. L., Ibrahim, D. A., Wren, T. A., & Barzdukas, A. (2014). Chronic Lack of Sleep is Associated With Increased Sports Injuries in Adolescent Athletes. Journal of Pediatric Orthopaedics, 34(2), 129–133. http://doi.org/10.1097/bpo.0000000000000151
Mougin, F., Bourdin, H., Simon-Rigaud, M., Didier, J., Toubin, G., & Kantelip, J. (1996). Effects of a Selective Sleep Deprivation on Subsequent Anaerobic Performance. International Journal of Sports Medicine, 17(02), 115–119. http://doi.org/10.1055/s-2007-972818
Oliver, S. J., Costa, R. J. S., Laing, S. J., Bilzon, J. L. J., & Walsh, N. P. (2009). One night of sleep deprivation decreases treadmill endurance performance. European Journal of Applied Physiology, 107(2), 155–161. http://doi.org/10.1007/s00421-009-1103-9
Pallesen, S., Gundersen, H. S., Kristoffersen, M., Bjorvatn, B., Thun, E., & Harris, A. (2017). The Effects of Sleep Deprivation on Soccer Skills. Perceptual and Motor Skills, 124(4), 812–829. http://doi.org/10.1177/0031512517707412
Reilly, T., & Piercy, M. (1994). The effect of partial sleep deprivation on weight-lifting performance. Ergonomics, 37(1), 107–115. http://doi.org/10.1080/00140139408963628
Rossa, K. R., Smith, S. S., Allan, A. C., & Sullivan, K. A. (2014). The Effects of Sleep Restriction on Executive Inhibitory Control and Affect in Young Adults. Journal of Adolescent Health, 55(2), 287–292. http://doi.org/10.1016/j.jadohealth.2013.12.034
Sargent, C., & Roach, G. D. (2016). Sleep duration is reduced in elite athletes following night-time competition. Chronobiology International, 33(6), 667–670. http://doi.org/10.3109/07420528.2016.1167715
Skein, M., Duffield, R., Edge, J., Short, M. J., & Mündel, T. (2011). Intermittent-Sprint Performance and Muscle Glycogen after 30 h of Sleep Deprivation. Medicine & Science in Sports & Exercise, 43(7), 1301–1311. http://doi.org/10.1249/mss.0b013e31820abc5a
Souissi, N., Sesboüé, B., Gauthier, A., Larue, J., & Davenne, D. (2003). Effects of one nights sleep deprivation on anaerobic performance the following day. European Journal of Applied Physiology, 89(3), 359–366. http://doi.org/10.1007/s00421-003-0793-7
Caia, J., Kelly, V. G., & Halson, S. L. (2017). The role of sleep in maximising performance in elite athletes. Sport, Recovery, and Performance, 151–167. http://doi.org/10.4324/9781315268149-11
Alley, J. R., Mazzochi, J. W., Smith, C. J., Morris, D. M., & Collier, S. R. (2015). Effects of Resistance Exercise Timing on Sleep Architecture and Nocturnal Blood Pressure. Journal of Strength and Conditioning Research, 29(5), 1378–1385. http://doi.org/10.1519/jsc.0000000000000750
Kovacevic, A., Mavros, Y., Heisz, J. J., & Singh, M. A. F. (2018). The effect of resistance exercise on sleep: A systematic review of randomized controlled trials. Sleep Medicine Reviews, 39, 52–68. http://doi.org/10.1016/j.smrv.2017.07.002
Herrick, J. E., Puri, S., & Richards, K. C. (2017). Resistance training does not alter same-day sleep architecture in institutionalized older adults. Journal of Sleep Research, 27(4). http://doi.org/10.1111/jsr.12590
Edinger, J. D., Morey, M. C., Sullivan, R. J., Higginbotham, M. B., Marsh, G. R., Dailey, D. S., & McCall, W. V. (1993). Aerobic fitness, acute exercise and sleep in older men. Sleep, 16(4), 351-359. https://doi.org/10.1093/sleep/16.4.351
King, A. C. (1997). Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA: The Journal of the American Medical Association, 277(1), 32–37. http://doi.org/10.1001/jama.277.1.32
Passos, G. S., Poyares, D., Santana, M. G., Garbuio, S. A., Tufik, S., & Mello, M. T. (2010). Effect of Acute Physical Exercise on Patients with Chronic Primary Insomnia. Journal of Clinical Sleep Medicine, 06(03), 270–275. http://doi.org/10.5664/jcsm.27825
Reid, K. J., Baron, K. G., Lu, B., Naylor, E., Wolfe, L., & Zee, P. C. (2010). Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Medicine, 11(9), 934–940. http://doi.org/10.1016/j.sleep.2010.04.014
Viana, V. A. R., Esteves, A. M., Boscolo, R. A., Grassmann, V., Santana, M. G., Tufik, S., & Mello, M. T. D. (2011). The effects of a session of resistance training on sleep patterns in the elderly. European Journal of Applied Physiology, 112(7), 2403–2408. http://doi.org/10.1007/s00421-011-2219-2
Herring, M., Kline, C., & Oconnor, P. (2015). Effects of Exercise Training On Self-reported Sleep Among Young Women with Generalized Anxiety Disorder (GAD). European Psychiatry, 30, 465. http://doi.org/10.1016/s0924-9338(15)31893-9
Kredlow, M. A., Capozzoli, M. C., Hearon, B. A., Calkins, A. W., & Otto, M. W. (2015). The effects of physical activity on sleep: a meta-analytic review. Journal of Behavioral Medicine, 38(3), 427–449. http://doi.org/10.1007/s10865-015-9617-6
Yang, P.-Y., Ho, K.-H., Chen, H.-C., & Chien, M.-Y. (2012). Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Journal of Physiotherapy, 58(3), 157–163. http://doi.org/10.1016/s1836-9553(12)70106-6
Kline, C. E., Sui, X., Hall, M. H., Youngstedt, S. D., Blair, S. N., Earnest, C. P., & Church, T. S. (2012). Dose–response effects of exercise training on the subjective sleep quality of postmenopausal women: exploratory analyses of a randomised controlled trial. BMJ Open, 2(4). http://doi.org/10.1136/bmjopen-2012-001044
Fairbrother, K., Cartner, B. W., Triplett, N., Morris, D. M., & Collier, S. R. (2011). The Effects of Aerobic Exercise Timing on Sleep Architecture. Medicine & Science in Sports & Exercise, 43(Suppl 1), 879. http://doi.org/10.1249/01.mss.0000402452.16375.20
Youngstedt, S. D., & Kline, C. E. (2006). Epidemiology of exercise and sleep. Sleep and Biological Rhythms, 4(3), 215–221. http://doi.org/10.1111/j.1479-8425.2006.00235.x
Stenholm, S., Head, J., Kivimäki, M., Hanson, L. L. M., Pentti, J., Rod, N. H., … Vahtera, J. (2018). Sleep Duration and Sleep Disturbances as Predictors of Healthy and Chronic Disease–Free Life Expectancy Between Ages 50 and 75: A Pooled Analysis of Three Cohorts. The Journals of Gerontology: Series A, 74(2), 204–210. http://doi.org/10.1093/gerona/gly01
Xiao, Q., Keadle, S. K., Hollenbeck, A. R., & Matthews, C. E. (2014). Sleep Duration and Total and Cause-Specific Mortality in a Large US Cohort: Interrelationships With Physical Activity, Sedentary Behavior, and Body Mass Index. American Journal of Epidemiology, 180(10), 997–1006. http://doi.org/10.1093/aje/kwu222
Reynolds, A. C., Dorrian, J., Liu, P. Y., Dongen, H. P. A. V., Wittert, G. A., Harmer, L. J., & Banks, S. (2012). Impact of Five Nights of Sleep Restriction on Glucose Metabolism, Leptin and Testosterone in Young Adult Men. PLoS ONE, 7(7). http://doi.org/10.1371/journal.pone.0041218
Åkerstedt, T., Palmblad, J., Torre, B. D. L., Marana, R., & Gillberg, M. (1980). Adrenocortical and Gonadal Steroids During Sleep Deprivation. Sleep, 3(1), 23–30. http://doi.org/10.1093/sleep/3.1.23
Cortés-Gallegos, V., Castañeda, G., Alonso, R., Sojo, I., Carranco, A., Cervantes, C., & Parra, A. (1983). Sleep Deprivation Reduces Circulating Androgens in Healthy Men. Archives of Andrology, 10(1), 33–37. http://doi.org/10.3109/01485018308990167
González-Santos, M. R., Gajá-Rodíguez, O. V., Alonso-Uriarte, R., Sojo-Aranda, I., & Cortés-Gallegos, V. (1989). Sleep Deprivation and Adaptive Hormonal Responses of Healthy Men. Archives of Andrology, 22(3), 203–207. http://doi.org/10.3109/01485018908986773
Penev, P. D. (2007). Association Between Sleep and Morning Testosterone Levels In Older Men. Sleep, 30(4), 427–432. http://doi.org/10.1093/sleep/30.4.427
Kloss, J. D., Perlis, M. L., Zamzow, J. A., Culnan, E. J., & Gracia, C. R. (2015). Sleep, sleep disturbance, and fertility in women. Sleep Medicine Reviews, 22, 78–87. http://doi.org/10.1016/j.smrv.2014.10.005
Mahoney, M. M. (2010). Shift Work, Jet Lag, and Female Reproduction. International Journal of Endocrinology, 2010, 1–9. http://doi.org/10.1155/2010/813764
Labyak, S., Lava, S., Turek, F., & Zee, P. (2002). Effects Of Shiftwork On Sleep And Menstrual Function In Nurses. Health Care for Women International, 23(6-7), 703–714. http://doi.org/10.1080/07399330290107449
Pal, L., Bevilacqua, K., Zeitlian, G., Shu, J., & Santoro, N. (2008). Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause, 15(6), 1086–1094. http://doi.org/10.1097/gme.0b013e3181728467
Axelsson, G., Rylander, R., & Molin, I. (1989). Outcome of pregnancy in relation to irregular and inconvenient work schedules. Occupational and Environmental Medicine, 46(6), 393–398. http://doi.org/10.1136/oem.46.6.393
Bisanti, L., Olsen, J., Basso, O., Thonneau, P., & Karmaus, W. (1996). Shift Work and Subfecundity: A European Multicenter Study. Journal of Occupational & Environmental Medicine, 38(4), 352–358. http://doi.org/10.1097/00043764-199604000-00012
Rossmanith, W. G. (1998). The impact of sleep on gonadotropin secretion. Gynecological Endocrinology, 12(6), 381–389. http://doi.org/10.3109/09513599809012840
Fernando, S., & Rombauts, L. (2014). Melatonin: shedding light on infertility? – a review of the recent literature. Journal of Ovarian Research, 7(1). http://doi.org/10.1186/s13048-014-0098-y
Rocha, C., Rato, L., Martins, A., Alves, M., & Oliveira, P. (2015). Melatonin and Male Reproductive Health: Relevance of Darkness and Antioxidant Properties. Current Molecular Medicine, 15(4), 299–311. http://doi.org/10.2174/1566524015666150505155530
Song, C., Peng, W., Yin, S., Zhao, J., Fu, B., Zhang, J., … Zhang, Y. (2016). Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Scientific Reports, 6(1). http://doi.org/10.1038/srep35165
Espino, J., Macedo, M., Lozano, G., Ortiz, Á., Rodríguez, C., Rodríguez, A. B., & Bejarano, I. (2019). Impact of Melatonin Supplementation in Women with Unexplained Infertility Undergoing Fertility Treatment. Antioxidants, 8(9), 338. http://doi.org/10.3390/antiox8090338
Tamura, H., Takasaki, A., Taketani, T., Tanabe, M., Kizuka, F., Lee, L., … Sugino, N. (2012). The role of melatonin as an antioxidant in the follicle. Journal of Ovarian Research, 5(1), 5. http://doi.org/10.1186/1757-2215-5-5
Saaresranta, T., & Polo, O. (2003). Sleep-disordered breathing and hormones. European Respiratory Journal, 22(1), 161–172. http://doi.org/10.1183/09031936.03.00062403
Cappuccio, F. P., Cooper, D., Delia, L., Strazzullo, P., & Miller, M. A. (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European Heart Journal, 32(12), 1484–1492. http://doi.org/10.1093/eurheartj/ehr007
Jansen, E. C., Dunietz, G. L., Tsimpanouli, M.-E., Guyer, H. M., Shannon, C., Hershner, S. D., … Baylin, A. (2018). Sleep, Diet, and Cardiometabolic Health Investigations: a Systematic Review of Analytic Strategies. Current Nutrition Reports, 7(4), 235–258. http://doi.org/10.1007/s13668-018-0240-3
Knutson, K. L., Cauter, E. V., Rathouz, P. J., Yan, L. L., Hulley, S. B., Liu, K., & Lauderdale, D. S. (2009). Association Between Sleep and Blood Pressure in Midlife. Archives of Internal Medicine, 169(11), 1055. http://doi.org/10.1001/archinternmed.2009.119
Besedovsky, L., Lange, T., & Born, J. (2011). Sleep and immune function. Pflügers Archiv – European Journal of Physiology, 463(1), 121–137. http://doi.org/10.1007/s00424-011-1044-0
Besedovsky, L., Lange, T., & Haack, M. (2019). The Sleep-Immune Crosstalk in Health and Disease. Physiological Reviews, 99(3), 1325–1380. http://doi.org/10.1152/physrev.00010.2018
Orr, W. C., Fass, R., Sundaram, S. S., & Scheimann, A. O. (2020). The effect of sleep on gastrointestinal functioning in common digestive diseases. The Lancet Gastroenterology & Hepatology, 5(6), 616–624. http://doi.org/10.1016/s2468-1253(19)30412-1
Tang, Y., Preuss, F., Turek, F. W., Jakate, S., & Keshavarzian, A. (2009). Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Medicine, 10(6), 597–603. http://doi.org/10.1016/j.sleep.2008.12.009
Chen, Y., Tan, F., Wei, L., Li, X., Lyu, Z., Feng, X., … Li, N. (2018). Sleep duration and the risk of cancer: a systematic review and meta-analysis including dose–response relationship. BMC Cancer, 18(1). http://doi.org/10.1186/s12885-018-5025-y
Almendros, I., Martinez-Garcia, M. A., Farré, R., & Gozal, D. (2020). Obesity, sleep apnea, and cancer. International Journal of Obesity, 44(8), 1653–1667. http://doi.org/10.1038/s41366-020-0549-z
Erren, T. C., Falaturi, P., Morfeld, P., Knauth, P., Reiter, R. J., & Piekarski, C. (2010). Shift Work and Cancer. Deutsches Aerzteblatt Online. http://doi.org/10.3238/arztebl.2010.0657
Bernert, R. A., Kim, J. S., Iwata, N. G., & Perlis, M. L. (2015). Sleep Disturbances as an Evidence-Based Suicide Risk Factor. Current Psychiatry Reports, 17(3). http://doi.org/10.1007/s11920-015-0554-4
Kim, J.-H., Park, E.-C., Cho, W.-H., Park, J.-Y., Choi, W.-J., & Chang, H.-S. (2013). Association between Total Sleep Duration and Suicidal Ideation among the Korean General Adult Population. Sleep, 36(10), 1563–1572. http://doi.org/10.5665/sleep.3058
Mccall, W. V., & Black, C. G. (2013). The Link Between Suicide and Insomnia: Theoretical Mechanisms. Current Psychiatry Reports, 15(9). http://doi.org/10.1007/s11920-013-0389-9
Li, S. X., Lam, S. P., Zhang, J., Yu, M. W. M., Chan, J. W. Y., Chan, C. S. Y., … Wing, Y.-K. (2016). Sleep Disturbances and Suicide Risk in an 8-Year Longitudinal Study of Schizophrenia-Spectrum Disorders. Sleep, 39(6), 1275–1282. http://doi.org/10.5665/sleep.5852
Littlewood, D. L., Gooding, P., Kyle, S. D., Pratt, D., & Peters, S. (2016). Understanding the role of sleep in suicide risk: qualitative interview study. BMJ Open, 6(8). http://doi.org/10.1136/bmjopen-2016-012113
Lin, H.-T., Lai, C.-H., Perng, H.-J., Chung, C.-H., Wang, C.-C., Chen, W.-L., & Chien, W.-C. (2018). Insomnia as an independent predictor of suicide attempts: a nationwide population-based retrospective cohort study. BMC Psychiatry, 18(1). http://doi.org/10.1186/s12888-018-1702-2
Freeman, D., Sheaves, B., Waite, F., Harvey, A. G., & Harrison, P. J. (2020). Sleep disturbance and psychiatric disorders. The Lancet Psychiatry, 7(7), 628–637. http://doi.org/10.1016/s2215-0366(20)30136-x
Benca, R. M. (1992). Sleep and Psychiatric Disorders. Archives of General Psychiatry, 49(8), 651. http://doi.org/10.1001/archpsyc.1992.01820080059010
Breslau, N., Roth, T., Rosenthal, L., & Andreski, P. (1996). Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young Adults. Biological Psychiatry, 39(6), 411–418. http://doi.org/10.1016/0006-3223(95)00188-3
Baglioni, C., Nanovska, S., Regen, W., Spiegelhalder, K., Feige, B., Nissen, C., … Riemann, D. (2016). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychological Bulletin, 142(9), 969–990. http://doi.org/10.1037/bul0000053
Goldstein, A. N., & Walker, M. P. (2014). The Role of Sleep in Emotional Brain Function. Annual Review of Clinical Psychology, 10(1), 679–708. http://doi.org/10.1146/annurev-clinpsy-032813-153716
Postuma, R. B., Iranzo, A., Hu, M., Högl, B., Boeve, B. F., Manni, R., … Pelletier, A. (2019). Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain, 142(3), 744–759. http://doi.org/10.1093/brain/awz030
Wintler, T., Schoch, H., Frank, M. G., & Peixoto, L. (2020). Sleep, brain development, and autism spectrum disorders: Insights from animal models. Journal of Neuroscience Research, 98(6), 1137–1149. http://doi.org/10.1002/jnr.24619
Shokri-Kojori, E., Wang, G.-J., Wiers, C. E., Demiral, S. B., Guo, M., Kim, S. W., … Volkow, N. D. (2018). β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proceedings of the National Academy of Sciences, 115(17), 4483–4488. http://doi.org/10.1073/pnas.1721694115
Mantovani, S., Smith, S. S., Gordon, R., & Osullivan, J. D. (2018). An overview of sleep and circadian dysfunction in Parkinsons disease. Journal of Sleep Research, 27(3). http://doi.org/10.1111/jsr.12673
Malhotra, R. K. (2018). Neurodegenerative Disorders and Sleep. Sleep Medicine Clinics, 13(1), 63–70. http://doi.org/10.1016/j.jsmc.2017.09.006
Huang, L.-B., Tsai, M.-C., Chen, C.-Y., & Hsu, S.-C. (2013). The Effectiveness of Light/Dark Exposure to Treat Insomnia in Female Nurses Undertaking Shift Work during the Evening/Night Shift. Journal of Clinical Sleep Medicine, 09(07), 641–646. http://doi.org/10.5664/jcsm.2824
Zhang, Y., & Papantoniou, K. (2019). Night shift work and its carcinogenicity. The Lancet Oncology, 20(10). http://doi.org/10.1016/s1470-2045(19)30578-9
Perry-Jenkins, M., Goldberg, A. E., Pierce, C. P., & Sayer, A. G. (2007). Shift Work, Role Overload, and the Transition to Parenthood. Journal of Marriage and Family, 69(1), 123–138. http://doi.org/10.1111/j.1741-3737.2006.00349.x
Rodziewicz TL, Hipskind JE. Medical Error Prevention. 2020 May 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 29763131. https://pubmed.ncbi.nlm.nih.gov/29763131/
Tanaka, K., Takahashi, M., Hiro, H., Kakinuma, M., Tanaka, M., Kamata, N., & Miyaoka, H. (2010). Differences in Medical Error Risk among Nurses Working Two- and Three-shift Systems at Teaching Hospitals: A Six-month Prospective Study. Industrial Health, 48(3), 357–364. http://doi.org/10.2486/indhealth.48.357
Admi H, Tzischinsky O, Epstein R, Herer P, Lavie P. Shift work in nursing: is it really a risk factor for nurses’ health and patients’ safety?. Nurs Econ. 2008;26(4):250-257. https://pubmed.ncbi.nlm.nih.gov/18777974/
Clendon, J., & Gibbons, V. (2015). 12h shifts and rates of error among nurses: A systematic review. International Journal of Nursing Studies, 52(7), 1231–1242. http://doi.org/10.1016/j.ijnurstu.2015.03.011
Hammadah, M., Kindya, B. R., Allard‐Ratick, M. P., Jazbeh, S., Eapen, D., Tang, W. W., & Sperling, L. (2017). Navigating air travel and cardiovascular concerns: Is the sky the limit?, Clinical Cardiology, 40 (9), 660–666. http://doi.org/10.1002/clc.22741
Lieber, B. A., Han, J., Appelboom, G., Taylor, B. E., Han, B., Agarwal, N., & Connolly, E. S. (2016). Association of Steroid Use with Deep Venous Thrombosis and Pulmonary Embolism in Neurosurgical Patients: A National Database Analysis. World Neurosurgery, 89, 126–132. http://doi.org/10.1016/j.wneu.2016.01.033
El-Menyar, A., Asim, M., & Al-Thani, H. (2017). Obesity Paradox in Patients With Deep Venous Thrombosis. Clinical and Applied Thrombosis/Hemostasis, 24(6), 986–992. http://doi.org/10.1177/1076029617727858
Klovaite, J., Benn, M., & Nordestgaard, B. G. (2014). Obesity as a causal risk factor for deep venous thrombosis: a Mendelian randomization study. Journal of Internal Medicine, 277(5), 573–584. http://doi.org/10.1111/joim.12299
Davies, H. O., Popplewell, M., Singhal, R., Smith, N., & Bradbury, A. W. (2016). Obesity and lower limb venous disease – The epidemic of phlebesity. Phlebology: The Journal of Venous Disease, 32(4), 227–233. http://doi.org/10.1177/0268355516649333
Liljeqvist, S., Helldén, A., Bergman, U., & Söderberg, M. (2008). Pulmonary embolism associated with the use of anabolic steroids. European Journal of Internal Medicine, 19(3), 214–215. http://doi.org/10.1016/j.ejim.2007.03.016
Linton MF, Yancey PG, Davies SS, Jerome WG (Jay), Linton EF, Vickers KC. The Role of Lipids and Lipoproteins in Atherosclerosis. In: De Groot LJ, Chrousos G, Dungan K, et al., eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. http://www.ncbi.nlm.nih.gov/books/NBK343489/.
Rescheduling of meals may ease the effects of jet lag. (2017). Nursing Standard, 31(48), 16–16. http://doi.org/10.7748/ns.31.48.16.s17
Ruscitto, C., & Ogden, J. (2016). The impact of an implementation intention to improve mealtimes and reduce jet lag in long-haul cabin crew. Psychology & Health, 32(1), 61–77. http://doi.org/10.1080/08870446.2016.1240174
Reid, K. J., & Abbott, S. M. (2015). Jet Lag and Shift Work Disorder. Sleep Medicine Clinics, 10(4), 523–535. http://doi.org/10.1016/j.jsmc.2015.08.006
Srinivasan, V., Spence, D. W., Pandi-Perumal, S. R., Trakht, I., & Cardinali, D. P. (2008). Jet lag: Therapeutic use of melatonin and possible application of melatonin analogs. Travel Medicine and Infectious Disease, 6(1-2), 17–28. http://doi.org/10.1016/j.tmaid.2007.12.002
Edwards, B. J., Atkinson, G., Waterhouse, J., Reilly, T., Godfrey, R., & Budgett, R. (2000). Use of melatonin in recovery from jet-lag following an eastward flight across 10 time-zones. Ergonomics, 43(10), 1501–1513. http://doi.org/10.1080/001401300750003934
Zee, P. C., & Goldstein, C. A. (2010). Treatment of Shift Work Disorder and Jet Lag. Current Treatment Options in Neurology, 12(5), 396–411. http://doi.org/10.1007/s11940-010-0090-9
https://www.nhlbi.nih.gov/health-topics/circadian-rhythm-disorders
Borodkin, K., & Dagan, Y. (2013). Diagnostic Algorithm for Circadian Rhythm Sleep Disorders. Encyclopedia of Sleep, 66–73. http://doi.org/10.1016/b978-0-12-378610-4.00284-9
Lockley, S. (2013). Special Considerations and Future Directions in Circadian Rhythm Sleep Disorders Diagnosis. Encyclopedia of Sleep, 138–149. http://doi.org/10.1016/b978-0-12-378610-4.00299-0
Crowley, S., & Youngstedt, S. (2013). Pathophysiology, Associations, and Consequences of Circadian Rhythm Sleep Disorder. Encyclopedia of Sleep, 16–21. http://doi.org/10.1016/b978-0-12-378610-4.00266-7
Franken, P., & Dijk, D.-J. (2009). Circadian clock genes and sleep homeostasis. European Journal of Neuroscience, 29(9), 1820–1829. http://doi.org/10.1111/j.1460-9568.2009.06723.x
Burgess, H. J., & Emens, J. S. (2016). Circadian-Based Therapies for Circadian Rhythm Sleep-Wake Disorders. Current Sleep Medicine Reports, 2(3), 158–165. http://doi.org/10.1007/s40675-016-0052-1
Jones, C. R., Huang, A. L., Ptáček, L. J., & Fu, Y.-H. (2013). Genetic basis of human circadian rhythm disorders. Experimental Neurology, 243, 28–33. http://doi.org/10.1016/j.expneurol.2012.07.012
Toh KL. Basic science review on circadian rhythm biology and circadian sleep disorders. Ann Acad Med Singap. 2008;37(8):662-668. https://pubmed.ncbi.nlm.nih.gov/18797559/
Farhud D, Aryan Z. Circadian Rhythm, Lifestyle and Health: A Narrative Review. Iran J Public Health. 2018;47(8):1068-1076. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6123576/
Dodson, E. R., & Zee, P. C. (2010). Therapeutics for Circadian Rhythm Sleep Disorders. Sleep Medicine Clinics, 5(4), 701–715. http://doi.org/10.1016/j.jsmc.2010.08.001
Zhu, L., & Zee, P. C. (2012). Circadian Rhythm Sleep Disorders. Neurologic Clinics, 30(4), 1167–1191. http://doi.org/10.1016/j.ncl.2012.08.011
Kim MJ, Lee JH, Duffy JF. Circadian Rhythm Sleep Disorders. J Clin Outcomes Manag. 2013;20(11):513-528. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4212693/
Zhong, G., Naismith, S. L., Rogers, N. L., & Lewis, S. J. G. (2011). Sleep-wake disturbances in common neurodegenerative diseases: A closer look at selected aspects of the neural circuitry. Journal of the Neurological Sciences, 307(1-2), 9–14. http://doi.org/10.1016/j.jns.2011.04.020
Dijk, D.-J., Boulos, Z., Eastman, C. I., Lewy, A. J., Campbell, S. S., & Terman, M. (1995). Light Treatment for Sleep Disorders: Consensus Report. Journal of Biological Rhythms, 10(2), 113–125. http://doi.org/10.1177/074873049501000204
Barion, A., & Zee, P. C. (2007). A clinical approach to circadian rhythm sleep disorders. Sleep Medicine, 8(6), 566–577. http://doi.org/10.1016/j.sleep.2006.11.017
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97-110. https://pubmed.ncbi.nlm.nih.gov/1027738/
Adan, A., & Almirall, H. (1991). Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Personality and Individual Differences, 12(3), 241–253. http://doi.org/10.1016/0191-8869(91)90110-w
Urbán, R., Magyaródi, T., & Rigó, A. (2011). Morningness-Eveningness, Chronotypes and Health-Impairing Behaviors in Adolescents. Chronobiology International, 28(3), 238–247. http://doi.org/10.3109/07420528.2010.549599
https://www.thewep.org/documentations/mctq
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. http://doi.org/10.1016/0165-1781(89)90047-4
Bastien, C. (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. http://doi.org/10.1016/s1389-9457(00)00065-4
Yang, M., Morin, C. M., Schaefer, K., & Wallenstein, G. V. (2009). Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Current Medical Research and Opinion, 25(10), 2487–2494. http://doi.org/10.1185/03007990903167415
Morin, C. M., Belleville, G., Bélanger, L., & Ivers, H. (2011). The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep, 34(5), 601–608. http://doi.org/10.1093/sleep/34.5.601
Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3(4):349-356. https://pubmed.ncbi.nlm.nih.gov/17694722/
Chokroverty S. Overview of sleep & sleep disorders. Indian J Med Res. 2010;131:126-140. https://pubmed.ncbi.nlm.nih.gov/20308738/
Pavlova, M. K., & Latreille, V. (2019). Sleep Disorders. The American Journal of Medicine, 132(3), 292–299. http://doi.org/10.1016/j.amjmed.2018.09.021
Olejniczak, P. W., & Fisch, B. J. (2003). Sleep disorders. Medical Clinics of North America, 87(4), 803–833. http://doi.org/10.1016/s0025-7125(03)00006-3
https://www.nhlbi.nih.gov/health-topics/sleep-apnea
https://clevemed.com/what-is-sleep-apnea/patient-sleep-apnea-screener/
Spicuzza, L., Caruso, D., & Maria, G. D. (2015). Obstructive sleep apnoea syndrome and its management. Therapeutic Advances in Chronic Disease, 6(5), 273–285. http://doi.org/10.1177/2040622315590318
Bixler, E. O., Vgontzas, A. N., Lin, H.-M., Liao, D., Calhoun, S., Fedok, F., … Graff, G. (2008). Blood Pressure Associated With Sleep-Disordered Breathing in a Population Sample of Children. Hypertension, 52(5), 841–846. http://doi.org/10.1161/hypertensionaha.108.116756
Campos, A. I., García-Marín, L. M., Byrne, E. M., Martin, N. G., Cuéllar-Partida, G., & Rentería, M. E. (2020). Insights into the aetiology of snoring from observational and genetic investigations in the UK Biobank. Nature Communications, 11(1). http://doi.org/10.1038/s41467-020-14625-1
Morgenthaler, T. I., Kagramanov, V., Hanak, V., & Decker, P. A. (2006). Complex Sleep Apnea Syndrome: Is It a Unique Clinical Syndrome? Sleep, 29(9), 1203–1209. http://doi.org/10.1093/sleep/29.9.1203
El-Ad, B., & Lavie, P. (2005). Effect of sleep apnea on cognition and mood. International Review of Psychiatry, 17(4), 277–282. http://doi.org/10.1080/09540260500104508
Morgenstern, M., Wang, J., Beatty, N., Batemarco, T., Sica, A. L., & Greenberg, H. (2014). Obstructive Sleep Apnea. Endocrinology and Metabolism Clinics of North America, 43(1), 187–204. http://doi.org/10.1016/j.ecl.2013.09.002
Sleep–Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research. (1999). Sleep, 22(5), 667–689. http://doi.org/10.1093/sleep/22.5.667
Ruehland, W. R., Rochford, P. D., O’Donoghue, F. J., Pierce, R. J., Singh, P., & Thornton, A. T. (2009). The New AASM Criteria for Scoring Hypopneas: Impact on the Apnea Hypopnea Index. Sleep, 32(2), 150–157. http://doi.org/10.1093/sleep/32.2.150
Selim, B. J., Koo, B. B., Qin, L., Jeon, S., Won, C., Redeker, N. S., … Yaggi, H. K. (2016). The Association between Nocturnal Cardiac Arrhythmias and Sleep-Disordered Breathing: The DREAM Study. Journal of Clinical Sleep Medicine, 12(06), 829–837. http://doi.org/10.5664/jcsm.5880
Ahmed, M. H. (2010). Obstructive sleep apnea syndrome and fatty liver: Association or causal link? World Journal of Gastroenterology, 16(34), 4243. http://doi.org/10.3748/wjg.v16.i34.4243
Singh, H., Pollock, R., Uhanova, J., Kryger, M., Hawkins, K., & Minuk, G. Y. (2005). Symptoms of Obstructive Sleep Apnea in Patients with Nonalcoholic Fatty Liver Disease. Digestive Diseases and Sciences, 50(12), 2338–2343. http://doi.org/10.1007/s10620-005-3058-y
Lawati, N. M. A., Patel, S. R., & Ayas, N. T. (2009). Epidemiology, Risk Factors, and Consequences of Obstructive Sleep Apnea and Short Sleep Duration. Progress in Cardiovascular Diseases, 51(4), 285–293. http://doi.org/10.1016/j.pcad.2008.08.001
Young, T. (2004). Risk Factors for Obstructive Sleep Apnea in Adults. Jama, 291(16), 2013. http://doi.org/10.1001/jama.291.16.2013
Yaggi, H. K., Concato, J., Kernan, W. N., Lichtman, J. H., Brass, L. M., & Mohsenin, V. (2005). Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. New England Journal of Medicine, 353(19), 2034–2041. http://doi.org/10.1056/nejmoa043104
Redline, S., Budhiraja, R., Kapur, V., Marcus, C. L., Mateika, J. H., Mehra, R., … Quan, A. S. F. (2007). The Scoring of Respiratory Events in Sleep: Reliability and Validity. Journal of Clinical Sleep Medicine, 03(02), 169–200. http://doi.org/10.5664/jcsm.26818
Basheer B, Hegde KS, Bhat SS, Umar D, Baroudi K. Influence of mouth breathing on the dentofacial growth of children: a cephalometric study. J Int Oral Health. 2014;6(6):50-55. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295456/
Ruhle, K. H., & Nilius, G. (2008). Mouth Breathing in Obstructive Sleep Apnea prior to and during Nasal Continuous Positive Airway Pressure. Respiration, 76(1), 40–45. http://doi.org/10.1159/000111806
Izu, S. C., Itamoto, C. H., Pradella-Hallinan, M., Pizarro, G. U., Tufik, S., Pignatari, S., & Fujita, R. R. (2010). Obstructive sleep apnea syndrome (OSAS) in mouth breathing children. Brazilian Journal of Otorhinolaryngology, 76(5), 552–556. http://doi.org/10.1590/s1808-86942010000500003
Lee, S. H., Choi, J. H., Shin, C., Lee, H. M., Kwon, S. Y., & Lee, S. H. (2007). How Does Open-Mouth Breathing Influence Upper Airway Anatomy? The Laryngoscope, 117(6), 1102–1106. http://doi.org/10.1097/mlg.0b013e318042aef7
Tuomilehto, H. P. I., Seppä, J. M., Partinen, M. M., Peltonen, M., Gylling, H., Tuomilehto, J. O. I., … Uusitupa, M. (2009). Lifestyle Intervention with Weight Reduction. American Journal of Respiratory and Critical Care Medicine, 179(4), 320–327. http://doi.org/10.1164/rccm.200805-669oc
https://www.cdc.gov/nchs/fastats/obesity-overweight.htm
Neill, A. M., Angus, S. M., Sajkov, D., & Mcevoy, R. D. (1997). Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 155(1), 199–204. http://doi.org/10.1164/ajrccm.155.1.9001312
Loord, H., & Hultcrantz, E. (2007). Positioner–a method for preventing sleep apnea. Acta Oto-Laryngologica, 127(8), 861–868. http://doi.org/10.1080/00016480601089390
Szollosi, I., Roebuck, T., Thompson, B., & Naughton, M. T. (2006). Lateral Sleeping Position Reduces Severity of Central Sleep Apnea / Cheyne-Stokes Respiration. Sleep, 29(8), 1045–1051. http://doi.org/10.1093/sleep/29.8.1045
Silverberg DS, Iaina A, Oksenberg A. Treating obstructive sleep apnea improves essential hypertension and quality of life. Am Fam Physician. 2002;65(2):229-236. https://pubmed.ncbi.nlm.nih.gov/11820487/
Aurora, R. N., Chowdhuri, S., Ramar, K., Bista, S. R., Casey, K. R., Lamm, C. I., … Tracy, S. L. (2012). The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses. Sleep, 35(1), 17–40. http://doi.org/10.5665/sleep.1580
Hsu, A. A. L., & Lo, C. (2003). Continuous positive airway pressure therapy in sleep apnoea. Respirology, 8(4), 447–454. http://doi.org/10.1046/j.1440-1843.2003.00494.x
Patel, S. R., White, D. P., Malhotra, A., Stanchina, M. L., & Ayas, N. T. (2003). Continuous Positive Airway Pressure Therapy for Treating gess in a Diverse Population With Obstructive Sleep Apnea. Archives of Internal Medicine, 163(5), 565. http://doi.org/10.1001/archinte.163.5.565
Sundaram, S., Lim, J., & Lasserson, T. J. (2005). Surgery for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews. http://doi.org/10.1002/14651858.cd001004.pub2
Chen, H., & Lowe, A. A. (2012). Updates in oral appliance therapy for snoring and obstructive sleep apnea. Sleep and Breathing, 17(2), 473–486. http://doi.org/10.1007/s11325-012-0712-4
Gaisl, T., Haile, S. R., Thiel, S., Osswald, M., & Kohler, M. (2019). Efficacy of pharmacotherapy for OSA in adults: A systematic review and network meta-analysis. Sleep Medicine Reviews, 46, 74–86. http://doi.org/10.1016/j.smrv.2019.04.009
Ohayon, M., Wickwire, E. M., Hirshkowitz, M., Albert, S. M., Avidan, A., Daly, F. J., … Vitiello, M. V. (2017). National Sleep Foundations sleep quality recommendations: first report. Sleep Health, 3(1), 6–19. http://doi.org/10.1016/j.sleh.2016.11.006
Youngstedt, S. D., Goff, E. E., Reynolds, A. M., Kripke, D. F., Irwin, M. R., Bootzin, R. R., … Jean-Louis, G. (2016). Has adult sleep duration declined over the last 50 years? Sleep Medicine Reviews, 28, 69–85. http://doi.org/10.1016/j.smrv.2015.08.004
Chaput, J.-P., Mcneil, J., Després, J.-P., Bouchard, C., & Tremblay, A. (2013). Seven to Eight Hours of Sleep a Night Is Associated with a Lower Prevalence of the Metabolic Syndrome and Reduced Overall Cardiometabolic Risk in Adults. PLoS ONE, 8(9). http://doi.org/10.1371/journal.pone.0072832
Wild, C. J., Nichols, E. S., Battista, M. E., Stojanoski, B., & Owen, A. M. (2018). Dissociable effects of self-reported daily sleep duration on high-level cognitive abilities. Sleep, 41(12). http://doi.org/10.1093/sleep/zsy182
Hirshkowitz, M., Whiton, K., Albert, S. M., Alessi, C., Bruni, O., Doncarlos, L., … Hillard, P. J. A. (2015). National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health, 1(1), 40–43. http://doi.org/10.1016/j.sleh.2014.12.010
Cappuccio, F. P., Delia, L., Strazzullo, P., & Miller, M. A. (2010). Sleep Duration and All-Cause Mortality: A Systematic Review and Meta-Analysis of Prospective Studies. Sleep, 33(5), 585–592. http://doi.org/10.1093/sleep/33.5.585
Gottlieb, D. J., Punjabi, N. M., Newman, A. B., Resnick, H. E., Redline, S., Baldwin, C. M., & Nieto, F. J. (2005). Association of Sleep Time With Diabetes Mellitus and Impaired Glucose Tolerance. Archives of Internal Medicine, 165(8), 863. http://doi.org/10.1001/archinte.165.8.863
Short, M. A., Agostini, A., Lushington, K., & Dorrian, J. (2015). A systematic review of the sleep, sleepiness, and performance implications of limited wake shift work schedules. Scandinavian Journal of Work, Environment & Health, 41(5), 425–440. http://doi.org/10.5271/sjweh.3509
Cappuccio, F. P., Taggart, F. M., Kandala, N.-B., Currie, A., Peile, E., Stranges, S., & Miller, M. A. (2008). Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults. Sleep, 31(5), 619–626. http://doi.org/10.1093/sleep/31.5.619
Mong, J. A., & Cusmano, D. M. (2016). Sex differences in sleep: impact of biological sex and sex steroids. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1688), 20150110. http://doi.org/10.1098/rstb.2015.0110
Krishnan, V., & Collop, N. A. (2006). Gender differences in sleep disorders. Current Opinion in Pulmonary Medicine, 12(6), 383–389. http://doi.org/10.1097/01.mcp.0000245705.69440.6a
Mehta, N., Shafi, F., & Bhat, A. (2015). Unique Aspects of Sleep in Women. Missouri medicine, 112(6), 430–434. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6168103/
Moline, M. L., Broch, L., & Zak, R. (2004). Sleep in women across the life cycle from adulthood through menopause. Medical Clinics of North America, 88(3), 705–736. http://doi.org/10.1016/j.mcna.2004.01.009
He, Y., Jones, C. R., Fujiki, N., Xu, Y., Guo, B., Holder, J. L., … Fu, Y.-H. (2009). The Transcriptional Repressor DEC2 Regulates Sleep Length in Mammals. Science, 325(5942), 866–870. http://doi.org/10.1126/science.1174443
Theorell-Haglöw, J., Berglund, L., Berne, C., & Lindberg, E. (2014). Both habitual short sleepers and long sleepers are at greater risk of obesity: a population-based 10-year follow-up in women. Sleep Medicine, 15(10), 1204–1211. http://doi.org/10.1016/j.sleep.2014.02.014
Mezick, E. J., Wing, R. R., & Mccaffery, J. M. (2014). Associations of self-reported and actigraphy-assessed sleep characteristics with body mass index and waist circumference in adults: moderation by gender. Sleep Medicine, 15(1), 64–70. http://doi.org/10.1016/j.sleep.2013.08.784
Kim, S. J. (2011). Relationship Between Weekend Catch-up Sleep and Poor Performance on Attention Tasks in Korean Adolescents. Archives of Pediatrics & Adolescent Medicine, 165(9), 806. http://doi.org/10.1001/archpediatrics.2011.128
Kim, C.-W., Choi, M.-K., Im, H.-J., Kim, O.-H., Lee, H.-J., Song, J., … Park, K.-H. (2012). Weekend catch-up sleep is associated with decreased risk of being overweight among fifth-grade students with short sleep duration. Journal of Sleep Research, 21(5), 546–551. http://doi.org/10.1111/j.1365-2869.2012.01013.x
Sun, W., Ling, J., Zhu, X., Lee, T. M.-C., & Li, S. X. (2019). Associations of weekday-to-weekend sleep differences with academic performance and health-related outcomes in school-age children and youths. Sleep Medicine Reviews, 46, 27–53. http://doi.org/10.1016/j.smrv.2019.04.003
Kang, S.-G., Lee, Y. J., Kim, S. J., Lim, W., Lee, H.-J., Park, Y.-M., … Hong, J. P. (2014). Weekend catch-up sleep is independently associated with suicide attempts and self-injury in Korean adolescents. Comprehensive Psychiatry, 55(2), 319–325. http://doi.org/10.1016/j.comppsych.2013.08.023
Zhao, M., Tuo, H., Wang, S., & Zhao, L. (2020). The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediators of Inflammation, 2020, 1–7. http://doi.org/10.1155/2020/3142874
Doherty, Madigan, Warrington, & Ellis. (2019). Sleep and Nutrition Interactions: Implications for Athletes. Nutrients, 11(4), 822. http://doi.org/10.3390/nu11040822
Sutanto CN, Wang MX, Tan D, Kim JE. Association of Sleep Quality and Macronutrient Distribution: A Systematic Review and Meta-Regression. Nutrients. 2020 Jan 2;12(1):126. doi: 10.3390/nu12010126. PMID: 31906452; PMCID: PMC7019667. https://pubmed.ncbi.nlm.nih.gov/31906452/
Peuhkuri, K., Sihvola, N., & Korpela, R. (2012). Diet promotes sleep duration and quality. Nutrition Research, 32(5), 309–319. http://doi.org/10.1016/j.nutres.2012.03.009
Afaghi, A., Oconnor, H., & Chow, C. M. (2007). High-glycemic-index carbohydrate meals shorten sleep onset. The American Journal of Clinical Nutrition, 85(2), 426–430. http://doi.org/10.1093/ajcn/85.2.426
Golem, D. L., Martin-Biggers, J. T., Koenings, M. M., Davis, K. F., & Byrd-Bredbenner, C. (2014). An Integrative Review of Sleep for Nutrition Professionals. Advances in Nutrition, 5(6), 742–759. http://doi.org/10.3945/an.114.006809
Grandner, M. A., Jackson, N., Gerstner, J. R., & Knutson, K. L. (2013). Sleep symptoms associated with intake of specific dietary nutrients. Journal of Sleep Research, 23(1), 22–34. http://doi.org/10.1111/jsr.12084
Porter, J., & Horne, J. (1981). Bed-time food supplements and sleep: Effects of different carbohydrate levels. Electroencephalography and Clinical Neurophysiology, 51(4), 426–433. http://doi.org/10.1016/0013-4694(81)90106-1
Halson, S. L. (2014). Sleep in Elite Athletes and Nutritional Interventions to Enhance Sleep. Sports Medicine, 44(S1), 13–23. http://doi.org/10.1007/s40279-014-0147-0
Rondanelli, M., Opizzi, A., Monteferrario, F., Antoniello, N., Manni, R., & Klersy, C. (2011). The Effect of Melatonin, Magnesium, and Zinc on Primary Insomnia in Long-Term Care Facility Residents in Italy: A Double-Blind, Placebo-Controlled Clinical Trial. Journal of the American Geriatrics Society, 59(1), 82–90. http://doi.org/10.1111/j.1532-5415.2010.03232.x
Tahara, Y., & Shibata, S. (2014). Chrono-biology, Chrono-pharmacology, and Chrono-nutrition. Journal of Pharmacological Sciences, 124(3), 320–335. http://doi.org/10.1254/jphs.13r06cr
Landolt, H.-P., Werth, E., Borbély, A. A., & Dijk, D.-J. (1995). Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Research, 675(1-2), 67–74. http://doi.org/10.1016/0006-8993(95)00040-w
Gottesmann, C. (2002). GABA mechanisms and sleep. Neuroscience, 111(2), 231–239. http://doi.org/10.1016/s0306-4522(02)00034-9
Campbell, S. S., Dawson, D., & Anderson, M. W. (1993). Alleviation of Sleep Maintenance Insomnia with Timed Exposure to Bright Light. Journal of the American Geriatrics Society, 41(8), 829–836. http://doi.org/10.1111/j.1532-5415.1993.tb06179.x
Zhao, J., Tian, Y., Nie, J., Xu, J., & Liu, D. (2012). Red Light and the Sleep Quality and Endurance Performance of Chinese Female Basketball Players. Journal of Athletic Training, 47(6), 673–678. http://doi.org/10.4085/1062-6050-47.6.08
Smolensky, M. H., Sackett-Lundeen, L. L., & Portaluppi, F. (2015). Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiology International, 32(8), 1029–1048. http://doi.org/10.3109/07420528.2015.1072002
Düzgün, G., & Akyol, A. D. (2017). Effect of Natural Sunlight on Sleep Problems and Sleep Quality of the Elderly Staying in the Nursing Home. Holistic Nursing Practice, 31(5), 295–302. http://doi.org/10.1097/hnp.0000000000000206
Valham, F., Sahlin, C., Stenlund, H., & Franklin, K. A. (2012). Ambient Temperature and Obstructive Sleep Apnea: Effects on Sleep, Sleep Apnea, and Morning Alertness. Sleep, 35(4), 513–517. http://doi.org/10.5665/sleep.1736
Okamoto-Mizuno, K., Tsuzuki, K., & Mizuno, K. (2004). Effects of mild heat exposure on sleep stages and body temperature in older men. International Journal of Biometeorology, 49(1). http://doi.org/10.1007/s00484-004-0209-3
St-Onge, M.-P., & Shechter, A. (2014). Sleep disturbances, body fat distribution, food intake and/or energy expenditure: pathophysiological aspects. Hormone Molecular Biology and Clinical Investigation, 17(1). http://doi.org/10.1515/hmbci-2013-0066
Chaput, J.-P., Després, J.-P., Bouchard, C., & Tremblay, A. (2008). The Association Between Sleep Duration and Weight Gain in Adults: A 6-Year Prospective Study from the Quebec Family Study. Sleep, 31(4), 517–523. http://doi.org/10.1093/sleep/31.4.517
Dekker, S. A., Noordam, R., Biermasz, N. R., Roos, A., Lamb, H. J., Rosendaal, F. R., … Mutsert, R. (2018). Habitual Sleep Measures are Associated with Overall Body Fat, and not Specifically with Visceral Fat, in Men and Women. Obesity, 26(10), 1651–1658. http://doi.org/10.1002/oby.22289
Tunnicliffe, J. M., Erdman, K. A., Reimer, R. A., Lun, V., & Shearer, J. (2008). Consumption of dietary caffeine and coffee in physically active populations: physiological interactions. Applied Physiology, Nutrition, and Metabolism, 33(6), 1301–1310. http://doi.org/10.1139/h08-124
Mahoney, C. R., Giles, G. E., Marriott, B. P., Judelson, D. A., Glickman, E. L., Geiselman, P. J., & Lieberman, H. R. (2019). Intake of caffeine from all sources and reasons for use by college students. Clinical Nutrition, 38(2), 668–675. http://doi.org/10.1016/j.clnu.2018.04.004
Binks, H., Vincent, G. E., Gupta, C., Irwin, C., & Khalesi, S. (2020). Effects of Diet on Sleep: A Narrative Review. Nutrients, 12(4), 936. http://doi.org/10.3390/nu12040936
Rao, T. P., Ozeki, M., & Juneja, L. R. (2015). In Search of a Safe Natural Sleep Aid. Journal of the American College of Nutrition, 34(5), 436–447. http://doi.org/10.1080/07315724.2014.926153
Abbasi B, Kimiagar M, Sadeghniiat K, Shirazi MM, Hedayati M, Rashidkhani B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J Res Med Sci. 2012 Dec;17(12):1161-9. https://pubmed.ncbi.nlm.nih.gov/23853635/
Aspy, D. J., Madden, N. A., & Delfabbro, P. (2018). Effects of Vitamin B6 (Pyridoxine) and a B Complex Preparation on Dreaming and Sleep. Perceptual and Motor Skills, 003151251877032. http://doi.org/10.1177/0031512518770326
Parazzini F. Resveratrol, tryptophanum, glycine and vitamin E: a nutraceutical approach to sleep disturbance and irritability in peri- and post-menopause. Minerva Ginecol. 2015;67(1):1-5. https://pubmed.ncbi.nlm.nih.gov/25660429/
Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry. 2004;65 Suppl 16:4-7. https://pubmed.ncbi.nlm.nih.gov/15575797/
Djeridane, Y., Touitou, Y. Chronic diazepam administration differentially affects melatonin synthesis in rat pineal and Harderian glands. Psychopharmacology154, 403–407 (2001). https://doi.org/10.1007/s002130000631
Betti L, Palego L, Demontis GC, Miraglia F, Giannaccini G. Hydroxyindole-O-methyltransferase (HIOMT) activity in the retina of melatonin-proficient mice. Heliyon. 2019;5(9):e02417. Published 2019 Sep 14. doi:10.1016/j.heliyon.2019.e02417
Haduch, A., Bromek, E., Wójcikowski, J., Gołembiowska, K., Daniel, W.. Melatonin Supports Serotonin Formation by Brain CYP2D. Drug Metabolism and DispositionMarch 1, 2016, 44 (3) 445-452; DOI: https://doi.org/10.1124/dmd.115.067413
Morton, D. J. (1987). Mechanism of Inhibition of Bovine Pineal Gland Hydroxyindole-O-Methyltransferase (EC 2.1.1.4) by Divalent Cations. Journal of Pineal Research, 4(3), 295–303. http://doi.org/10.1111/j.1600-079x.1987.tb00867.x
Markova-Car, E. P., Jurišić, D., Ilić, N., & Pavelić, S. K. (2014). Running for time: circadian rhythms and melanoma. Tumor Biology, 35(9), 8359–8368. http://doi.org/10.1007/s13277-014-1904-2
Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v-115. doi:10.1007/978-3-642-19683-6_1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3422784/
Chalupsky, K., Kračun, D., Kanchev, I., Bertram, K., & Görlach, A. (2015). Folic Acid Promotes Recycling of Tetrahydrobiopterin and Protects Against Hypoxia-Induced Pulmonary Hypertension by Recoupling Endothelial Nitric Oxide Synthase. Antioxidants & Redox Signaling, 23(14), 1076–1091. http://doi.org/10.1089/ars.2015.6329
Dianzani, I., Sanctis, L. D., Smooker, P. M., Gough, T. J., Alliaudi, C., Brusco, A., … Cotton, R. G. H. (1998). Dihydropteridine reductase deficiency: Physical structure of the QDPR gene, identification of two new mutations and genotype–phenotype correlations. Human Mutation, 12(4), 267–273. http://doi.org/10.1002/(sici)1098-1004(1998)12:4<267::aid-humu8>3.0.co;2-c
Nichol, C. A., Lee, C. L., Edelstein, M. P., Chao, J. Y., & Duch, D. S. (1983). Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proceedings of the National Academy of Sciences, 80(6), 1546–1550. http://doi.org/10.1073/pnas.80.6.1546
Titus, F., Dávalos, A., Alom, J., & Codina, A. (1986). 5-Hydroxytryptophan versus Methysergide in the Prophylaxis of Migraine. European Neurology, 25(5), 327–329. http://doi.org/10.1159/000116030
Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev. 1998;3(4):271-280. https://pubmed.ncbi.nlm.nih.gov/9727088/
Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3865832/
Valadas, J. S., Esposito, G., Vandekerkhove, D., Miskiewicz, K., Deaulmerie, L., Raitano, S., … Verstreken, P. (2018). ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease. Neuron, 98(6). http://doi.org/10.1016/j.neuron.2018.05.022
Chung SY, Moriyama T, Uezu E, et al. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J Nutr. 1995;125(6):1484-1489. doi:10.1093/jn/125.6.1484 https://pubmed.ncbi.nlm.nih.gov/7782901/
Montgomery, P., Burton, J. R., Sewell, R. P., Spreckelsen, T. F., & Richardson, A. J. (2014). Fatty acids and sleep in UK children: subjective and pilot objective sleep results from the DOLAB study – a randomized controlled trial. Journal of Sleep Research, 23(4), 364–388. http://doi.org/10.1111/jsr.12135
Alzoubi, K. H., Mayyas, F., & Zamzam, H. I. A. (2019). Omega-3 fatty acids protects against chronic sleep-deprivation induced memory impairment. Life Sciences, 227, 1–7. http://doi.org/10.1016/j.lfs.2019.04.028
Nasehi, M., Mosavi-Nezhad, S.-M., Khakpai, F., & Zarrindast, M.-R. (2018). The role of omega-3 on modulation of cognitive deficiency induced by REM sleep deprivation in rats. Behavioural Brain Research, 351, 152–160. http://doi.org/10.1016/j.bbr.2018.06.002
Jahangard, L., Sadeghi, A., Ahmadpanah, M., Holsboer-Trachsler, E., Bahmani, D. S., Haghighi, M., & Brand, S. (2018). Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders – Results from a double-blind, randomized and placebo-controlled clinical trial. Journal of Psychiatric Research, 107, 48–56. http://doi.org/10.1016/j.jpsychires.2018.09.016
Scorza, F. A., Cavalheiro, E. A., Scorza, C. A., Galduróz, J. C. F., Tufik, S., & Andersen, M. L. (2013). Sleep Apnea and Inflammation – Getting a Good Night’s Sleep with Omega-3 Supplementation. Frontiers in Neurology, 4. http://doi.org/10.3389/fneur.2013.00193
Hansen, A. L., Dahl, L., Olson, G., Thornton, D., Graff, I. E., Frøyland, L., … Pallesen, S. (2014). Fish Consumption, Sleep, Daily Functioning, and Heart Rate Variability. Journal of Clinical Sleep Medicine, 10(05), 567–575. http://doi.org/10.5664/jcsm.3714
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., … Nedergaard, M. (2013). Sleep Drives Metabolite Clearance from the Adult Brain. Science, 342(6156), 373–377. http://doi.org/10.1126/science.1241224
Varshavsky, A. (2012). Augmented generation of protein fragments during wakefulness as the molecular cause of sleep: a hypothesis. Protein Science, 21(11), 1634–1661. http://doi.org/10.1002/pro.2148
Mackiewicz, M., Shockley, K. R., Romer, M. A., Galante, R. J., Zimmerman, J. E., Naidoo, N., … Pack, A. I. (2007). Macromolecule biosynthesis: a key function of sleep. Physiological Genomics, 31(3), 441–457. http://doi.org/10.1152/physiolgenomics.00275.2006
Scharf, M. T., Naidoo, N., Zimmerman, J. E., & Pack, A. I. (2008). The energy hypothesis of sleep revisited. Progress in Neurobiology, 86(3), 264–280. http://doi.org/10.1016/j.pneurobio.2008.08.003
Horne, J. (2009). REM sleep, energy balance and ‘optimal foraging.’ Neuroscience & Biobehavioral Reviews, 33(3), 466–474. http://doi.org/10.1016/j.neubiorev.2008.12.002
Berger, R. J., & Phillips, N. H. (1995). Energy conservation and sleep. Behavioural Brain Research, 69(1-2), 65–73. http://doi.org/10.1016/0166-4328(95)00002-b
Benington, J. H., & Heller, H. C. (1995). Restoration of brain energy metabolism as the function of sleep. Progress in Neurobiology, 45(4), 347–360. http://doi.org/10.1016/0301-0082(94)00057-o
Abel, T., Havekes, R., Saletin, J. M., & Walker, M. P. (2013). Sleep, Plasticity and Memory from Molecules to Whole-Brain Networks. Current Biology, 23(17). http://doi.org/10.1016/j.cub.2013.07.025
Rasch, B., & Born, J. (2013). About Sleeps Role in Memory. Physiological Reviews, 93(2), 681–766. http://doi.org/10.1152/physrev.00032.2012
Cirelli, C., & Tononi, G. (2008). Is Sleep Essential? PLoS Biology, 6(8). http://doi.org/10.1371/journal.pbio.0060216
Siegel, J. M. (2005). Clues to the functions of mammalian sleep. Nature, 437(7063), 1264–1271. http://doi.org/10.1038/nature04285
Campbell, S. S., & Tobler, I. (1984). Animal sleep: A review of sleep duration across phylogeny. Neuroscience & Biobehavioral Reviews, 8(3), 269–300. http://doi.org/10.1016/0149-7634(84)90054-x
Tobler, I. (1995). Is sleep fundamentally different between mammalian species? Behavioural Brain Research, 69(1-2), 35–41. http://doi.org/10.1016/0166-4328(95)00025-o
Tononi, G., & Cirelli, C. (2006). Sleep function and synaptic homeostasis. Sleep Medicine Reviews, 10(1), 49–62. http://doi.org/10.1016/j.smrv.2005.05.002
Dongen, H. P. A. V., Vitellaro, K. M., & Dinges, D. F. (2005). Individual Differences in Adult Human Sleep and Wakefulness: Leitmotif for a Research Agenda. Sleep, 28(4), 479–498. http://doi.org/10.1093/sleep/28.4.479
Vyazovskiy, V. V., & Delogu, A. (2014). NREM and REM Sleep. The Neuroscientist, 20(3), 203–219. http://doi.org/10.1177/1073858413518152
Mignot, E. (2008). Why We Sleep: The Temporal Organization of Recovery. PLoS Biology, 6(4). http://doi.org/10.1371/journal.pbio.0060106
Siegel, J. M. (2009). Sleep viewed as a state of adaptive inactivity. Nature Reviews Neuroscience, 10(10), 747–753. http://doi.org/10.1038/nrn2697
Horne, J. (2000). REM sleep — by default? Neuroscience & Biobehavioral Reviews, 24(8), 777–797. http://doi.org/10.1016/s0149-7634(00)00037-3
Baran, B., Pace-Schott, E. F., Ericson, C., & Spencer, R. M. C. (2012). Processing of Emotional Reactivity and Emotional Memory over Sleep. Journal of Neuroscience, 32(3), 1035–1042. http://doi.org/10.1523/jneurosci.2532-11.2012
Tononi, G., & Cirelli, C. (2014). Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron, 81(1), 12–34. http://doi.org/10.1016/j.neuron.2013.12.025
Li, J., Vitiello, M. V., & Gooneratne, N. S. (2018). Sleep in Normal Aging. Sleep Medicine Clinics, 13(1), 1–11. http://doi.org/10.1016/j.jsmc.2017.09.001
Murillo-Rodriguez, E., Arias-Carrion, O., Zavala-Garcia, A., Sarro-Ramirez, A., & Huitron-Resendiz, S. (2012). Basic Sleep Mechanisms: An Integrative Review. Central Nervous System Agents in Medicinal Chemistry, 12(1), 38–54. http://doi.org/10.2174/187152412800229107
Weber, F. D. (2018). Sleep: Eye-Opener Highlights Sleep’s Organization. Current Biology, 28(5). http://doi.org/10.1016/j.cub.2018.01.054
Yetton, B. D., Mcdevitt, E. A., Cellini, N., Shelton, C., & Mednick, S. C. (2018). Quantifying sleep architecture dynamics and individual differences using big data and Bayesian networks. Plos One, 13(4). http://doi.org/10.1371/journal.pone.0194604
Colrain, I. M., Nicholas, C. L., & Baker, F. C. (2014). Alcohol and the sleeping brain. Handbook of Clinical Neurology Alcohol and the Nervous System, 415–431. http://doi.org/10.1016/b978-0-444-62619-6.00024-0
Zisapel, N. (2018). New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. British Journal of Pharmacology, 175(16), 3190–3199. http://doi.org/10.1111/bph.14116
Tosini, G., Baba, K., Hwang, C. K., & Iuvone, P. M. (2012). Melatonin: An underappreciated player in retinal physiology and pathophysiology. Experimental Eye Research, 103, 82–89. http://doi.org/10.1016/j.exer.2012.08.009
Blasiak, J., Reiter, R. J., & Kaarniranta, K. (2016). Melatonin in Retinal Physiology and Pathology: The Case of Age-Related Macular Degeneration. Oxidative Medicine and Cellular Longevity, 2016, 1–12. http://doi.org/10.1155/2016/6819736
Bellingham, J., Chaurasia, S. S., Melyan, Z., Liu, C., Cameron, M. A., Tarttelin, E. E., … Lucas, R. J. (2006). Evolution of Melanopsin Photoreceptors: Discovery and Characterization of a New Melanopsin in Nonmammalian Vertebrates. PLoS Biology, 4(8). http://doi.org/10.1371/journal.pbio.0040254
Zaidi, F. H., Hull, J. T., Peirson, S. N., Wulff, K., Aeschbach, D., Gooley, J. J., … Lockley, S. W. (2007). Short-Wavelength Light Sensitivity of Circadian, Pupillary, and Visual Awareness in Humans Lacking an Outer Retina. Current Biology, 17(24), 2122–2128. http://doi.org/10.1016/j.cub.2007.11.034
Legates, T. A., Altimus, C. M., Wang, H., Lee, H.-K., Yang, S., Zhao, H., … Hattar, S. (2012). Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature, 491(7425), 594–598. http://doi.org/10.1038/nature11673
Sikka, G., Hussmann, G. P., Pandey, D., Cao, S., Hori, D., Park, J. T., … Berkowitz, D. E. (2014). Melanopsin mediates light-dependent relaxation in blood vessels. Proceedings of the National Academy of Sciences, 111(50), 17977–17982. http://doi.org/10.1073/pnas.1420258111
Buhr, E. D., Yoo, S.-H., & Takahashi, J. S. (2010). Temperature as a Universal Resetting Cue for Mammalian Circadian Oscillators. Science, 330(6002), 379–385. http://doi.org/10.1126/science.1195262
Robbins, R., Grandner, M. A., Buxton, O. M., Hale, L., Buysse, D. J., Knutson, K. L., … Jean-Louis, G. (2019). Sleep myths: an expert-led study to identify false beliefs about sleep that impinge upon population sleep health practices. Sleep Health, 5(4), 409–417. http://doi.org/10.1016/j.sleh.2019.02.002
Damiola, F. (2000). Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & Development, 14(23), 2950–2961. http://doi.org/10.1101/gad.183500
Abel, T., Havekes, R., Saletin, J. M., & Walker, M. P. (2013). Sleep, Plasticity and Memory from Molecules to Whole-Brain Networks. Current Biology, 23(17). http://doi.org/10.1016/j.cub.2013.07.025
Muzet, A., Ehrhart, J., Candas, V., Libert, J. P., & Vogt, J. J. (1983). Rem Sleep and Ambient Temperature in Man. International Journal of Neuroscience, 18(1-2), 117–125. http://doi.org/10.3109/00207458308985885
Saini, C., Morf, J., Stratmann, M., Gos, P., & Schibler, U. (2012). Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes & Development, 26(6), 567–580. http://doi.org/10.1101/gad.183251.111
Franco P, Szliwowski H, Dramaix M, Kahn A. Influence of ambient temperature on sleep characteristics and autonomic nervous control in healthy infants. Sleep. 2000 May 1;23(3):401-7. https://pubmed.ncbi.nlm.nih.gov/10811384/
Libert JP, Candas V, Muzet A, Ehrhart J. Thermoregulatory adjustments to thermal transients during slow wave sleep and REM sleep in man. J Physiol (Paris). 1982;78(3):251-7 https://pubmed.ncbi.nlm.nih.gov/7166740/
Palca, J. W., Walker, J. M., & Berger, R. J. (1986). Thermoregulation, metabolism, and stages of sleep in cold-exposed men. Journal of Applied Physiology, 61(3), 940–947. http://doi.org/10.1152/jappl.1986.61.3.940
Lack. (2009). Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nature and Science of Sleep, 1. http://doi.org/10.2147/nss.s6234
Samson, D. R., Crittenden, A. N., Mabulla, I. A., Mabulla, A. Z. P., & Nunn, C. L. (2017). Chronotype variation drives night-time sentinel-like behaviour in hunter–gatherers. Proceedings of the Royal Society B: Biological Sciences, 284(1858), 20170967. http://doi.org/10.1098/rspb.2017.0967
Walker, R. J., Kribs, Z. D., Christopher, A. N., Shewach, O. R., & Wieth, M. B. (2014). Age, the Big Five, and time-of-day preference: A mediational model. Personality and Individual Differences, 56, 170–174. http://doi.org/10.1016/j.paid.2013.09.003
Bjorness, T., & Greene, R. (2009). Adenosine and Sleep. Current Neuropharmacology, 7(3), 238–245. http://doi.org/10.2174/157015909789152182
Dworak, M., Diel, P., Voss, S., Hollmann, W., & Strüder, H. (2007). Intense exercise increases adenosine concentrations in rat brain: Implications for a homeostatic sleep drive. Neuroscience, 150(4), 789–795. http://doi.org/10.1016/j.neuroscience.2007.09.062
Rainnie, D., Grunze, H., Mccarley, R., & Greene, R. (1994). Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science, 263(5147), 689–692. http://doi.org/10.1126/science.8303279
Daly, J. W., Shi, D., Nikodijevic, O., & Jacobson, K. A. (1994). The role of adenosine receptors in the central action of caffeine. Pharmacopsychoecologia, 7(2), 201–213. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4373791/

